Growth characteristics, redox potential changes and proton motive force generation in Thermus scotoductus K1 during growth on various carbon sources
- PMID: 39628724
- PMCID: PMC11609421
- DOI: 10.3934/microbiol.2024045
Growth characteristics, redox potential changes and proton motive force generation in Thermus scotoductus K1 during growth on various carbon sources
Abstract
The extremophile microorganism Thermus scotoductus primarily exhibits aerobic metabolism, though some strains are capable of anaerobic growth, utilizing diverse electron acceptors. We focused on the T. scotoductus K1 strain, exploring its aerobic growth and metabolism, responses to various carbon sources, and characterization of its bioenergetic and physiological properties. The strain grew on different carbon sources, depending on their concentration and the medium's pH, demonstrating adaptability to acidic environments (pH 6.0). It was shown that 4 g L-1 glucose inhibited the specific growth rate by approximately 4.8-fold and 5.6-fold compared to 1 g L-1 glucose at pH 8.5 and pH 6.0, respectively. However, this inhibition was not observed in the presence of fructose, galactose, lactose, and starch. Extracellular and intracellular pH variations were mainly alkalifying during growth. At pH 6.0, the membrane potential (ΔΨ) was lower for all carbon sources compared to pH 8.5. The proton motive force (Δp) was lower only during growth on lactose due to the difference in the transmembrane proton gradient (ΔpH). Moreover, at pH 6.0 during growth on lactose, a positive Δp was detected, indicating the cells' ability to employ a unique energy-conserving strategy. Taken together, these findings concluded that Thermus scotoductus K1 exhibits different growth and bioenergetic properties depending on the carbon source, which can be useful for biotechnological applications. These findings offer valuable insights into how bacterial cells function under high-temperature conditions, which is essential for applying bioenergetics knowledge in future biotechnological advancements.
Keywords: Thermus scotoductus; aerobic metabolism; pH; proton motive force; temperature.
© 2024 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures

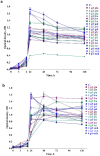

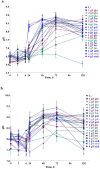


References
-
- Sharp R, Williams R. Thermus Species. New York: Springer; 1995. - DOI
-
- Kristjánsson JK, Hjörleifsdóttir S, Marteinsson VT, et al. Thermus scotoductus, sp. nov., a pigment-producing thermophilic bacterium from hot tap water in iceland and including Thermus sp. X-1. Syst Appl Microbiol. 1994;17:44–50. doi: 10.1016/S0723-2020(11)80030-5. - DOI
-
- Babák L, Šupinová P, Burdychová R. Growth models of Thermus aquaticus and Thermus scotoductus. Acta Univ Agric Silvic Mendel Brun. 2013;60:19–26. doi: 10.11118/actaun201260050019. - DOI
LinkOut - more resources
Full Text Sources
