Non-invasive nanoscale imaging of protein micro- and nanocrystals for screening crystallization conditions
- PMID: 39628883
- PMCID: PMC11611282
- DOI: 10.1107/S1600576724010124
Non-invasive nanoscale imaging of protein micro- and nanocrystals for screening crystallization conditions
Abstract
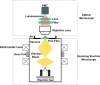
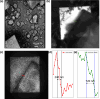
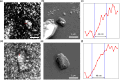
Crystallography has been the routine technique for studying high-resolution structures of proteins for over five decades. A major bottleneck in structure determination of macromolecules is obtaining crystals of a size and quality suitable for single-crystal X-ray crystallography experiments. Many challenging proteins either fail to grow into crystals or fail to grow into crystals of a size suitable for obtaining high-resolution structures using conventional X-ray crystallography. When it comes to smaller crystals, they can be used either for seeding to get larger crystals or for serial crystallography and electron diffraction for obtaining the structures. For both purposes, a limiting step is to non-invasively image such small crystals of sub-micrometre dimensions and to screen the conditions where such crystals prevail. Here we use cathodoluminescence-based (CL-based) nanoscopy to image protein nanocrystals. We show that crystals of micrometre and submicrometre dimensions can be non-invasively imaged by the CL-based nanoscope. The results presented here demonstrate the feasibility of non-invasive imaging of protein crystals with sub-100 nm resolution.
Keywords: cathodoluminescence; nanocrystals; nanoscopy; protein crystal screening.
© Krishna Prasad Khakurel et al. 2024.
Figures


References
-
- Bischak, C. G., Wai, R. B., Cherqui, C., Busche, J. A., Quillin, S. C., Hetherington, C. L., Wang, Z., Aiello, C. D., Schlom, D. G., Aloni, S., Ogletree, D. F., Masiello, D. J. & Ginsberg, N. S. (2017). ACS Nano, 11, 10583–10590. - PubMed
-
- Bodenstaff, E. R., Hoedemaeker, F. J., Kuil, M. E., de Vrind, H. P. M. & Abrahams, J. P. (2002). Acta Cryst. D58, 1901–1906. - PubMed
-
- Canfield, R. E. (1963). J. Biol. Chem.238, 2698–2707. - PubMed
-
- Chapman, H. N., Fromme, P., Barty, A., White, T. A., Kirian, R. A., Aquila, A., Hunter, M. S., Schulz, J., DePonte, D. P., Weierstall, U., Doak, R. B., Maia, F. R. N. C., Martin, A. V., Schlichting, I., Lomb, L., Coppola, N., Shoeman, R. L., Epp, S. W., Hartmann, R., Rolles, D., Rudenko, A., Foucar, L., Kimmel, N., Weidenspointner, G., Holl, P., Liang, M., Barthelmess, M., Caleman, C., Boutet, S., Bogan, M. J., Krzywinski, J., Bostedt, C., Bajt, S., Gumprecht, L., Rudek, B., Erk, B., Schmidt, C., Hömke, A., Reich, C., Pietschner, D., Strüder, L., Hauser, G., Gorke, H., Ullrich, J., Herrmann, S., Schaller, G., Schopper, F., Soltau, H., Kühnel, K., Messerschmidt, M., Bozek, J. D., Hau-Riege, S. P., Frank, M., Hampton, C. Y., Sierra, R. G., Starodub, D., Williams, G. J., Hajdu, J., Timneanu, N., Seibert, M. M., Andreasson, J., Rocker, A., Jönsson, O., Svenda, M., Stern, S., Nass, K., Andritschke, R., Schröter, C., Krasniqi, F., Bott, M., Schmidt, K. E., Wang, X., Grotjohann, I., Holton, J. M., Barends, T. R. M., Neutze, R., Marchesini, S., Fromme, R., Schorb, S., Rupp, D., Adolph, M., Gorkhover, T., Andersson, I., Hirsemann, H., Potdevin, G., Graafsma, H., Nilsson, B. & Spence, J. C. H. (2011). Nature, 470, 73–77.
LinkOut - more resources
Full Text Sources
