A Singular Case Analysis: Lamotrigine-Associated Stevens-Johnson Syndrome
- PMID: 39628963
- PMCID: PMC11614506
- DOI: 10.1155/crcc/4835223
A Singular Case Analysis: Lamotrigine-Associated Stevens-Johnson Syndrome
Abstract
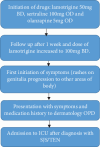
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) is an immune complex-mediated hypersensitivity reaction linked as an adverse side effect to many drugs. There have been case reports of similar incidences in Nepal related to various medications. Here, we present a case of a 29-year-old lady who developed a generalized erythematous rash over her body and erosion of the oral mucous membrane. Two weeks back she gave a history of initiation of lamotrigine, olanzapine, and sertraline. Given the strong association between SJS and lamotrigine, and the usual presentation being within the first 8 weeks of exposure to susceptible medications; she was diagnosed as SJS/TEN induced by lamotrigine. On April 1, 2024, she was admitted to the ICU at KIST MCTH. All the medicines were withheld, and she was managed with corticosteroids and antihistamines. She improved significantly within 7 days. Early identification of SJS, discontinuation of triggering medicines, and prompt initiation of supportive therapy improved the prognosis.
Keywords: Stevens–Johnson syndrome (SJS); adverse drug reaction (ADR); antiepileptic drugs; case report; lamotrigine; toxic epidermal necrolysis (TEN).
Copyright © 2024 Albin Joshi et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- WHO_EDM_QSM_2002.2.pdf. [cited 2024 May 11]. https://iris.who.int/bitstream/handle/10665/67378/WHO_EDM_QSM_2002.2.pdf....
-
- Jha N., Bajracharya O., Namgyal T. Prevalence of adverse drug reactions with commonly prescribed drugs in different hospitals of Kathmandu valley. Kathmandu University Medical Journal (KUMJ) . 2007;5(4):504–510. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

