Efficacy and safety of lebrikizumab for the treatment of moderate-to-severe atopic dermatitis: a systematic review and meta-analysis
- PMID: 39629076
- PMCID: PMC11611541
- DOI: 10.3389/fphar.2024.1429709
Efficacy and safety of lebrikizumab for the treatment of moderate-to-severe atopic dermatitis: a systematic review and meta-analysis
Abstract
Background: Lebrikizumab, an IL-13 immunomodulator, has shown recommendable effectiveness and safety in clinical studies for the treatment of moderate-to-severe atopic dermatitis (AD) in adolescents and adults.
Objective: To evaluate the efficacy and safety of lebrikizumab in the treatment of moderate-to-severe AD through a meta-analysis.
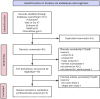
Methods: PubMed, Embase, Web of Science, Medline, and ClinicalTrials.gov databases were searched up to 8 August 2023. Randomized clinical trials of lebrikizumab treatment for moderate-to-severe AD were included by screening titles, abstracts, and papers.
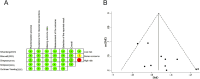
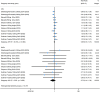
Results: Five studies involving 1,551 patients with AD were identified. Pooled analysis revealed significant improvements in the Eczema Area and Severity Index (EASI) score (SMD = -0.527; 95% CI = [-0.617, -0.436]), Investigator's Global Assessment (IGA) score (RR = 2.122; 95% CI = [1.803, 2.496]), Body Surface Area (BSA) score (SMD = -0.608; 95% CI = [-1.099, -0.118]), SCORing Atopic Dermatitis (SCORAD) score (SMD = -0.441; 95% CI = [-0.633, -0.250]). Moreover, Pruritus Numeric Rating Scale (P-NRS) score, Patient-oriented Eczema Measure (POEM) scores, Sleep-loss score and Dermatology Life Quality Index (DLQI) scores showed similar results. Adverse events (AEs) (RR = 0.984; 95% CI = [0.907, 1.068]) for lebrikizumab showed no statistically significant difference compared to placebo, with similar results for serious adverse events (SAEs) (RR = 0.748; 95% CI = [0.410, 1.364]).
Conclusion: This meta-analysis reveals that lebrikizumab has higher efficacy and safety in the treatment of moderate-to-severe AD, with the 250 mg Q2W dosage regimen appearing to be more advantageous.
Keywords: atopic dermatitis; efficacy; lebrikizumab; meta-analysis; safety.
Copyright © 2024 Lin, Luo, Zhuo, Chen, Zhang and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

