Comparison of the effects of deferasirox film-coated tablets (Jadenu®) and deferasirox dispersible tablets (Exjade®) in patients with beta thalassemia major: a preliminary report of the effects on the satisfaction, convenience, cardiac/liver MRI T2*, serum ferritin level, and biochemical profiles
- PMID: 39629077
- PMCID: PMC11611563
- DOI: 10.3389/fphar.2024.1438611
Comparison of the effects of deferasirox film-coated tablets (Jadenu®) and deferasirox dispersible tablets (Exjade®) in patients with beta thalassemia major: a preliminary report of the effects on the satisfaction, convenience, cardiac/liver MRI T2*, serum ferritin level, and biochemical profiles
Abstract
Background: Deferasirox (DFX) is a once-daily oral iron chelator with proven dose-dependent efficacy in patients with thalassemia major (TM). The reason for switching from DFX dispersible tablets (Exjade®) to DFX film-coated tablets (Jadenu®) was intolerance. Many patients also reported that deferasirox® did not taste good. In this study, we compared the effect of Jadenu® and Exjade® on satisfaction, convenience, cardiac/liver MRI T2*, serum ferritin levels, and biochemical profiles in patients with thalassemia major.
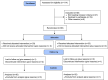
Method: Sixty-two patients with thalassemia over 2 years of age, who had iron overload indicated by chelation therapy, were randomly divided into two groups. The first group (n = 32) is treated with Exjade®, and the second group (n = 30) is treated with Jadenu®. Laboratory investigations included alkaline phosphatase (ALK), alanine transferase (ALT), aspartate transferase (AST), and serum ferritin levels. Cardiac/liver MRI T2* levels and patient satisfaction and convenience, were assessed before and 1 year after starting therapy.
Results: The study found that 53.3% of Jadenu® patients were satisfied with the taste of the medication compared to only 12.5% of Exjade® patients, which was statistically significant (p = 0.001). Additionally, 40% of Jadenu® patients were satisfied with the ease of taking the medication compared to 28.1% of Exjade® patients, and again, the difference was statistically significant (p = 0.047). A comparison of the cardiac MRI T2* levels between the two studied groups showed no significant difference (p = 0.851).
Conclusion: Jadenu® offers patients an improved formulation that can be taken on an empty stomach, has a better taste, and presents fewer gastrointestinal tolerability concerns. Overall, patient satisfaction is higher with Jadenu®, which may improve adherence and reduce the frequency and severity of complications associated with iron overload. This, in turn, may help mitigate cardiovascular and hepatic complications from iron overload in the long term.
Clinical trial registration: https://irct.behdasht.gov.ir/search/result?query=IRCT20210830052346N1.
Keywords: convenience; deferasirox; heart; iron overload; liver; satisfaction; thalassemia major.
Copyright © 2024 Mobinikhaledi, Falahati, Tajerian, Hashiani, Ghaffari and Ghasemi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources