TRIM4 enhances small-molecule-induced neddylated-degradation of CORO1A for triple negative breast cancer therapy
- PMID: 39629122
- PMCID: PMC11610137
- DOI: 10.7150/thno.97662
TRIM4 enhances small-molecule-induced neddylated-degradation of CORO1A for triple negative breast cancer therapy
Abstract
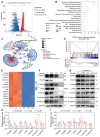
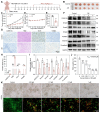
Background: As a critical member of the Coronin family, Coronin 1A (CORO1A) plays a crucial role in the progression of triple-negative breast cancer (TNBC). However, CORO1A is typically considered "undruggable" due to its smooth surface and complex protein-protein interactions (PPIs). Molecular glues have emerged as one of the most effective strategies to rapidly degrade such "undruggable" targets. Neddylation, an emerging approach, has shown promise in targeting pathogenic proteins for degradation through the NEDD8 pathway, making the degradation of CORO1A an attractive pharmacological strategy. Methods: A phenotypic drug screening strategy coupled with multi-omics approaches was utilized to rapidly identify a molecular glue degrader for CORO1A and to uncover the associated mechanisms. The Omics and Text-based Target Enrichment and Ranking (OTTER) tools, co-immunoprecipitation (Co-IP) assay, mass spectrometry, and the separation of phases-based protein interaction reporter (SPPIER) method were employed to explore the interaction between Aurovertin B (AB) and CORO1A via TRIM4. The pharmacological effects of AB were assessed using TNBC patient-derived organoids (PDOs) and 3D bioprinting models. Results: We identified AB as a previously undisclosed molecular glue that significantly promotes the neddylation and proteasomal degradation of CORO1A via TRIM4, an atypical E3 ligase. Notably, the degradation of CORO1A markedly inhibited various cellular processes and exerted robust antitumor effects in TNBC PDOs and 3D bioprinting models. Conclusions: Our findings underscore the critical role of CORO1A in TNBC and lay a crucial foundation for the development of innovative drugs based on molecular glue technology.
Keywords: Aurovertin B; CORO1A; Molecular glues; Neddylation; Triple-negative breast cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J Clin. 2021;71:209–49. - PubMed
-
- Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. Bmj-Brit Med J. 2023;381:e071674. - PubMed
-
- Torres QS, Canha-Borges A, Oliveira MJ, Sarmento B, Castro F. Special Issue: Nanotherapeutics in Women's Health Emerging Nanotechnologies for Triple-Negative Breast Cancer Treatment. Small. 2023;20:e2300666. - PubMed
-
- Jaiswal A, Kaushik N, Choi EH, Kaushik NK. Functional impact of non-coding RNAs in high-grade breast carcinoma: Moving from resistance to clinical applications: A comprehensive review. Biochim Biophys Acta Rev Cancer. 2023;1878:188915. - PubMed
-
- Xavier CP, Eichinger L, Fernandez MP, Morgan RO, Clemen CS. Evolutionary and functional diversity of coronin proteins. Subcell Biochem. 2008;48:98–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

