Molecular imaging along the heart-kidney axis
- PMID: 39629123
- PMCID: PMC11610144
- DOI: 10.7150/thno.102552
Molecular imaging along the heart-kidney axis
Abstract
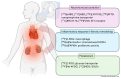
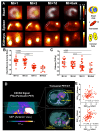
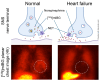
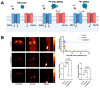
Cardiorenal syndrome (CRS) involves bidirectional crosstalk between the failing heart and the kidneys. Depending on the primum movens (primary cardiac or renal injury), systems-based interactions in the secondary affected organ may include pro-fibrotic signaling, overzealous inflammation, impaired nerve integrity or overactivity of specific renal transporters mediating glucose absorption. Those pathophysiological pillars can be investigated by molecular imaging using SPECT or PET agents. Targeted whole-body molecular imaging may allow for a) systems-based analysis along the heart-kidney axis, b) may provide prognostic information on longitudinal organ-based functional decline or c) may be used for guidance of reparative intervention based on peak activation identified on PET (paradigm of cardiorenal theranostics). We will discuss the current state of translational molecular imaging for CRS, along with future clinical aspects in the field.
Keywords: PET; cardiorenal; cardiorenal syndrome; heart-kidney axis; molecular imaging; organ-organ interaction; renocardiac; theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: R.A.W.: speaker honoraria from Novartis/AAA and PentixaPharm, advisory board work for Novartis/AAA and Bayer. All other authors declare no conflict of interest.
Figures

References
-
- Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL. et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e840–e78. - PubMed
-
- Grenier N, Merville P, Combe C. Radiologic imaging of the renal parenchyma structure and function. Nat Rev Nephrol. 2016;12:348–59. - PubMed
-
- Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7:703–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

