Salivary diagnostics: opportunities and challenges
- PMID: 39629130
- PMCID: PMC11610148
- DOI: 10.7150/thno.100600
Salivary diagnostics: opportunities and challenges
Abstract
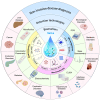
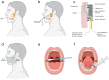
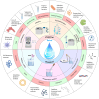
Saliva contains a diverse array of biomarkers indicative of various diseases. Saliva testing has been a major advancement towards non-invasive point-of-care diagnosis with clinical significance. However, there are challenges associated with salivary diagnosis from sample treatment and standardization. This review highlights the biomarkers in saliva and their role in identifying relevant diseases. It provides an overview and discussion about the current practice of saliva collection and processing, and advancements in saliva detection systems from in vitro methods to wearable oral devices. The review also addresses challenges in saliva diagnostics and proposes solutions, aiming to offer a comprehensive understanding and practical guidance for improving saliva-based detection in clinical diagnosis. Saliva diagnosis provides a rapid, effective, and safe alternative to traditional blood and urine tests for screening large populations and enhancing infectious disease diagnosis and surveillance. It meets the needs of various fields such as disease management, drug screening, and personalized healthcare with advances in saliva detection systems offering high sensitivity, fast response times, portability, and automation. Standardization of saliva collection, treatment, biomarker discovery, and detection between different laboratories needs to be implemented to obtain reliable salivary diagnosis in clinical practice.
Keywords: Salivary diagnostics; biomarkers; non-invasive detection; point-of-care testing; result standardization.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment.Appl Biochem Biotechnol. 2012 Nov;168(6):1718-27. doi: 10.1007/s12010-012-9891-5. Epub 2012 Sep 13. Appl Biochem Biotechnol. 2012. PMID: 22971835 Review.
-
Point-of-care platforms for salivary diagnostics.Chin J Dent Res. 2012;15(1):7-15. Chin J Dent Res. 2012. PMID: 22866276
-
Sample-to-answer salivary miRNA testing: New frontiers in point-of-care diagnostic technologies.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 May-Jun;16(3):e1969. doi: 10.1002/wnan.1969. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 38783564 Free PMC article. Review.
-
Saliva diagnostics - Current views and directions.Exp Biol Med (Maywood). 2017 Mar;242(5):459-472. doi: 10.1177/1535370216681550. Epub 2016 Dec 8. Exp Biol Med (Maywood). 2017. PMID: 27903834 Free PMC article. Review.
-
Saliva as a Diagnostic Tool for Systemic Diseases-A Narrative Review.Medicina (Kaunas). 2025 Jan 30;61(2):243. doi: 10.3390/medicina61020243. Medicina (Kaunas). 2025. PMID: 40005360 Free PMC article. Review.
Cited by
-
Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients.Int J Mol Sci. 2025 May 16;26(10):4784. doi: 10.3390/ijms26104784. Int J Mol Sci. 2025. PMID: 40429927 Free PMC article.
-
The presence and relative abundance of salivary Fusobacterium nucleatum are not associated with colorectal cancer: a systematic review and meta-analysis.Sci Rep. 2025 Jul 10;15(1):24815. doi: 10.1038/s41598-025-07465-w. Sci Rep. 2025. PMID: 40640262 Free PMC article.
-
Saliva urea nitrogen for detection of kidney disease in adults: A meta-analysis of diagnostic test accuracy.PLoS One. 2025 May 29;20(5):e0324251. doi: 10.1371/journal.pone.0324251. eCollection 2025. PLoS One. 2025. PMID: 40440346 Free PMC article.
-
Saliva metabolomics: a non-invasive frontier for diagnosing and managing oral diseases.J Transl Med. 2025 May 24;23(1):582. doi: 10.1186/s12967-025-06587-z. J Transl Med. 2025. PMID: 40413543 Free PMC article. Review.
-
Salivary Metabolomics Discloses Metabolite Signatures of Oral Leukoplakia with and Without Dysplasia.Int J Mol Sci. 2025 Jul 7;26(13):6519. doi: 10.3390/ijms26136519. Int J Mol Sci. 2025. PMID: 40650295 Free PMC article.
References
-
- To KKW, Yip CCY, Lai CYW. et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

