Hybrid-integrated devices for mimicking malignant brain tumors ("tumor-on-a-chip") for in vitro development of targeted drug delivery and personalized therapy approaches
- PMID: 39629230
- PMCID: PMC11611596
- DOI: 10.3389/fmed.2024.1452298
Hybrid-integrated devices for mimicking malignant brain tumors ("tumor-on-a-chip") for in vitro development of targeted drug delivery and personalized therapy approaches
Abstract
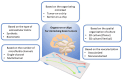
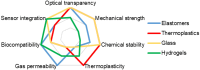
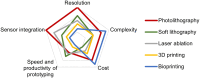
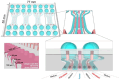
Acute and requiring attention problem of oncotheranostics is a necessity for the urgent development of operative and precise diagnostics methods, followed by efficient therapy, to significantly reduce disability and mortality of citizens. A perspective way to achieve efficient personalized treatment is to use methods for operative evaluation of the individual drug load, properties of specific tumors and the effectiveness of selected therapy, and other actual features of pathology. Among the vast diversity of tumor types-brain tumors are the most invasive and malignant in humans with poor survival after diagnosis. Among brain tumors glioblastoma shows exceptionally high mortality. More studies are urgently needed to understand the risk factors and improve therapy approaches. One of the actively developing approaches is the tumor-on-a-chip (ToC) concept. This review examines the achievements of recent years in the field of ToC system developments. The basics of microfluidic chips technologies are considered in the context of their applications in solving oncological problems. Then the basic principles of tumors cultivation are considered to evaluate the main challengers in implementation of microfluidic devices, for growing cell cultures and possibilities of their treatment and observation. The main achievements in the culture types diversity approaches and their advantages are being analyzed. The modeling of angiogenesis and blood-brain barrier (BBB) on a chip, being a principally important elements of the life system, were considered in detail. The most interesting examples and achievements in the field of tumor-on-a-chip developments have been presented.
Keywords: blood-brain barrier; brain tumor; microfluidic devices; organ-on-a-chip; tumor-on-a-chip.
Copyright © 2024 Zimina, Sitkov, Gareev, Mikhailova, Combs and Shevtsov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Current overview of induced pluripotent stem cell-based blood-brain barrier-on-a-chip.World J Stem Cells. 2023 Jun 26;15(6):632-653. doi: 10.4252/wjsc.v15.i6.632. World J Stem Cells. 2023. PMID: 37424947 Free PMC article.
-
BBB-on-a-Chip: Modeling Functional Human Blood-Brain Barrier by Mimicking 3D Brain Angiogenesis Using Microfluidic Chip.Methods Mol Biol. 2022;2492:251-263. doi: 10.1007/978-1-0716-2289-6_14. Methods Mol Biol. 2022. PMID: 35733049
-
Implementation of blood-brain barrier on microfluidic chip: Recent advance and future prospects.Ageing Res Rev. 2023 Jun;87:101921. doi: 10.1016/j.arr.2023.101921. Epub 2023 Mar 31. Ageing Res Rev. 2023. PMID: 37004842 Review.
-
Recent Developments in Glioblastoma-On-A-Chip for Advanced Drug Screening Applications.Small. 2025 Jan;21(1):e2405511. doi: 10.1002/smll.202405511. Epub 2024 Nov 13. Small. 2025. PMID: 39535474 Review.
-
Lab-on-a-Chip Platforms as Tools for Drug Screening in Neuropathologies Associated with Blood-Brain Barrier Alterations.Biomolecules. 2021 Jun 21;11(6):916. doi: 10.3390/biom11060916. Biomolecules. 2021. PMID: 34205550 Free PMC article. Review.
Cited by
-
Roles and Impacts of Integrative Medical Interventions in Central Nervous System Tumor Treatment: Multi-Technology Convergence and the Paradigm Shift Toward Functional Reconstruction.CNS Neurosci Ther. 2025 Jul;31(7):e70516. doi: 10.1111/cns.70516. CNS Neurosci Ther. 2025. PMID: 40654157 Free PMC article. Review.
References
-
- Cancer Prevention Science Communication. WCRF (2023). Available at: https://www.wcrf.org/world-cancer-day-2023-working-to-prevent-cancer-wor... (accessed February 13, 2024).
Publication types
LinkOut - more resources
Full Text Sources

