A Vaccine Against Fibroblast Activation Protein Improves Murine Cardiac Fibrosis by Preventing the Accumulation of Myofibroblasts
- PMID: 39629565
- PMCID: PMC11692786
- DOI: 10.1161/CIRCRESAHA.124.325017
A Vaccine Against Fibroblast Activation Protein Improves Murine Cardiac Fibrosis by Preventing the Accumulation of Myofibroblasts
Abstract
Background: Myofibroblasts are primary cells involved in chronic response-induced cardiac fibrosis. Fibroblast activation protein (FAP) is a relatively specific marker of activated myofibroblasts and a potential target molecule. This study aimed to clarify whether a vaccine targeting FAP could eliminate myofibroblasts in chronic cardiac stress model mice and reduce cardiac fibrosis.
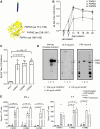
Methods: We coadministered a FAP peptide vaccine with a cytosine-phosphate-guanine (CpG) K3 oligonucleotide adjuvant to male C57/BL6J mice and confirmed an elevation in the anti-FAP antibody titer. After continuous angiotensin II and phenylephrine administration for 28 days, we evaluated the degree of cardiac fibrosis and the number of myofibroblasts in cardiac tissues.
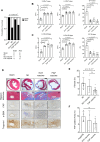
Results: We found that cardiac fibrosis was significantly decreased in the FAP-vaccinated mice compared with the angiotensin II and phenylephrine control mice (3.45±1.11% versus 8.62±4.79%; P=4.59×10-3) and that the accumulation of FAP-positive cells was also significantly decreased, as indicated by FAP immunohistochemical staining (4077±1746 versus 7327±1741 cells/mm2; FAP vaccine versus angiotensin II and phenylephrine control; P=6.67×10-3). No systemic or organ-specific inflammation due to antibody-dependent cell cytotoxicity induced by the FAP vaccine was observed. Although the transient activation of myofibroblasts has an important role in maintaining the structural robustness in the process of tissue repair, the FAP vaccine showed no adverse effects in myocardial infarction and skin injury models.
Conclusions: Our study demonstrates the FAP vaccine can be a therapeutic tool for cardiac fibrosis.
Keywords: fibrosis; myofibroblasts; vaccines.
Conflict of interest statement
The Department of Health Development and Medicine is an endowed department supported by Anges, Daicel, and FunPep. The Department of Clinical Gene Therapy is financially supported by Novartis, AnGes, Shionogi, Boeringher, Fancl, Saisei Mirai Clinics, Rohto and Funpep. The Department of Gene & Stem Cell Regenerative Therapy is an endowed department supported by AS medical support. H. Nakagami is a scientific advisor and stockholder of Funpep. R. Morishita is a scientific advisor and stockholder of FunPep and Anges. A. Temna and M. Toyoura are employees of FunPep. They did not have any role in the data analysis, decision to publish, or preparation of the manuscript. All other authors report no conflicts.
Figures




Comment in
-
Targeting Cardiac Fibrosis With a Vaccine Against Fibroblast Activation Protein.Circ Res. 2025 Jan 3;136(1):41-43. doi: 10.1161/CIRCRESAHA.124.325804. Epub 2025 Jan 2. Circ Res. 2025. PMID: 39745992 No abstract available.
References
-
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558 - PubMed
-
- Kurose H, Mangmool S. Myofibroblasts and inflammatory cells as players of cardiac fibrosis. Arch Pharm Res. 2016;39:1100–1113. doi: 10.1007/s12272-016-0809-6 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

