Loss of function of VCP/TER94 causes neurodegeneration
- PMID: 39629589
- PMCID: PMC11698056
- DOI: 10.1242/dmm.050359
Loss of function of VCP/TER94 causes neurodegeneration
Abstract
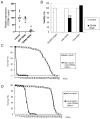
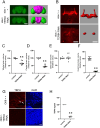
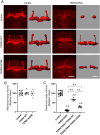
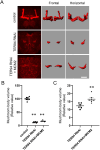
Variants in several genes are linked to human frontotemporal lobar degeneration (FTLD) associated with TDP43- and/or ubiquitin-positive inclusions. However, it is not yet clear whether the underlying mechanism is a gain-of-function or a loss-of-function one. To answer this question, we used Drosophila expressing double-stranded RNA against the FTLD-associated gene TER94 (an ortholog of VCP/p97) and found that the knockdown (KD) of this gene caused premature lethality, reduction in brain volume and alterations in the morphology of mushroom bodies. The changes caused by TER94 KD were rescued by wild-type TER94 but not by the human disease-linked A229E mutant, indicating that this mutant causes loss of function. Alterations were also observed in pupal brains and were partially rescued by co-expression of Mcm2, which is involved in control of the cell cycle, suggesting that dysregulation of neuronal proliferation caused the phenotypes. TER94 KD also caused the disappearance of TBPH (an ortholog of TDP43/TARDBP) from nuclei. These data from Drosophila genetics suggest that VCP-linked FTLD is caused by loss-of-function of VCP.
Keywords: Drosophila; Frontotemporal dementia; Neurodegeneration; TDP43; TER94; VCP.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Arimoto, E., Kawashima, Y., Choi, T., Unagami, M., Akiyama, S., Tomizawa, M., Yano, H., Suzuki, E. and Sone, M. (2020). Analysis of a cellular structure observed in the compound eyes of Drosophila white; yata mutants and white mutants. Biol. Open 9, bio047043. 10.1242/bio.047043 - DOI - PMC - PubMed
-
- Badadani, M., Nalbandian, A., Watts, G. D., Vesa, J., Kitazawa, M., Su, H., Tanaja, J., Dec, E., Wallace, D. C., Mukherjee, J.et al. (2010). VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS ONE 5, e13183. 10.1371/journal.pone.0013183 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

