E-52862-A selective sigma-1 receptor antagonist, in peripheral neuropathic pain: Two randomized, double-blind, phase 2 studies in patients with chronic postsurgical pain and painful diabetic neuropathy
- PMID: 39629978
- PMCID: PMC11616472
- DOI: 10.1002/ejp.4755
E-52862-A selective sigma-1 receptor antagonist, in peripheral neuropathic pain: Two randomized, double-blind, phase 2 studies in patients with chronic postsurgical pain and painful diabetic neuropathy
Abstract
Background: We report the efficacy and safety of E-52862-a selective, sigma-1 receptor antagonist-from phase 2, randomized, proof-of-concept studies in patients with moderate-to-severe, neuropathic, chronic postsurgical pain (CPSP) and painful diabetic neuropathy (PDN).
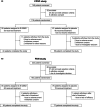
Methods: Adult patients (CPSP [N = 116]; PDN [N = 163]) were randomized at a 1:1 ratio to 4 weeks of treatment with E-52862 (CPSP [n = 55]; PDN [n = 85]) or placebo (CPSP [n = 61]; PDN [n = 78]) orally once daily. Pain intensity scores were measured using a numerical pain rating scale from 0 (no pain) to 10 (worst pain imaginable). The primary analysis population comprised patients who received study drug with ≥1 baseline and on-treatment observation (full analysis set).
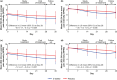
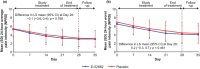
Results: In CPSP, mean baseline average pain was 6.2 for E-52862 vs. 6.5 for placebo. Week 4 mean change from baseline (CFB) for average pain was -1.6 for E-52862 vs. -0.9 for placebo (least squares mean difference [LSMD]: -0.9; p = 0.029). In PDN, mean baseline average pain was 5.3 for E-52862 vs. 5.4 for placebo. Week 4 mean CFB for average pain was -2.2 for E-52862 vs. -2.1 for placebo (LSMD: -0.1; p = 0.766). Treatment-emergent adverse events (TEAEs) were reported in 90.9% of E-52862-treated patients vs. 76.7% of placebo-treated patients in CPSP and 34.1% vs. 26.9% in PDN. Serious TEAEs occurred in CPSP only: E-52862: 5.5%; placebo: 6.7%.
Conclusions: E-52862 demonstrated superior relief of CPSP vs. placebo after 4 weeks. Reductions in pain intensity were seen in PDN with E-52862; high placebo response rates may have prevented differentiation between treatments. E-52862 had acceptable tolerability in both populations.
Significance statement: These proof-of-concept studies validate the mode of action of E-52862, a selective sigma-1 receptor antagonist. In CPSP, E-52862 resulted in clinically meaningful pain relief. In PDN, reductions in pain intensity were seen with E-52862; high placebo response rates may have prevented differentiation between E-52862 and placebo. These findings are clinically relevant given that neuropathic pain is highly incapacitating, lacking effective treatments and representing a significant unmet medical need, and support further development of sigma-1 receptor antagonists for peripheral neuropathic pain.
© 2024 Esteve Pharmaceuticals, S.A and The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Conflict of interest statement
RG, VM, FJM, AM‐P, FN‐R and LC report no conflicts of interest. JC, AM, MS and AV are employees of ESTEVE Pharmaceuticals S.A. (Barcelona, Spain). DB reports consulting fees from Bayer AG, ESTEVE Pharmaceuticals S.A. and Grünenthal Ltd., and honoraria from Grünenthal Ltd.
Figures

References
-
- Abadias, M. , Escriche, M. , Vaqué, A. , Sust, M. , & Encina, G. (2013). Safety, tolerability and pharmacokinetics of single and multiple doses of a novel sigma‐1 receptor antagonist in three randomized phase I studies. British Journal of Clinical Pharmacology, 75, 103–117. 10.1111/j.1365-2125.2012.04333.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

