GLP-1 Receptor Agonists Alleviate Diabetic Kidney Injury via β-Klotho-Mediated Ferroptosis Inhibition
- PMID: 39630101
- PMCID: PMC11775532
- DOI: 10.1002/advs.202409781
GLP-1 Receptor Agonists Alleviate Diabetic Kidney Injury via β-Klotho-Mediated Ferroptosis Inhibition
Abstract
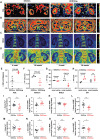
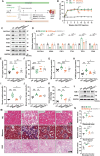
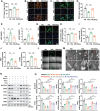
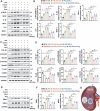
Semaglutide (Smg), a GLP-1 receptor agonist (GLP-1RA), shows renal protective effects in patients with diabetic kidney disease (DKD). However, the exact underlying mechanism remains elusive. This study employs transcriptome sequencing and identifies β-Klotho (KLB) as the critical target responsible for the role of Smg in kidney protection. Smg treatment alleviates diabetic kidney injury by inhibiting ferroptosis in patients, animal models, and HK-2 cells. Notably, Smg treatment significantly increases the mRNA expression of KLB through the activation of the cyclic adenosine monophosphate (cAMP) signaling pathway, specifically through the phosphorylation of protein kinase A (PKA) and cAMP-response element-binding protein (CREB). Subsequently, the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway is activated, reprograming the key metabolic processes of ferroptosis such as iron metabolism, fatty acid synthesis, and the antioxidant response against lipid peroxidation. Suppression of ferroptosis by Smg further attenuates renal inflammation and fibrosis. This work highlights the potential of GLP-1RAs and KLB targeting as promising therapeutic approaches for DKD management.
Keywords: GLP‐1 receptor agonist; diabetic kidney disease; ferroptosis; semaglutide; β‐Klotho.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B. B., Stein C., Basit A., Chan J. C. N., Mbanya J. C., Pavkov M. E., Ramachandaran A., Wild S. H., James S., Herman W. H., Zhang P., Bommer C., Kuo S., Boyko E. J., Magliano D. J., Diabetes Res. Clin. Pract. 2022, 183, 109119. - PMC - PubMed
-
- Selby N. M., Taal M. W., Diabetes Obes. Metab. 2020, 22, 3.
-
- Thomas M. C., Cooper M. E., Zimmet P., Nat. Rev. Nephrol. 2016, 12, 73. - PubMed
-
- Gribble F. M., Reimann F., Nat. Metab. 2021, 3, 142. - PubMed
MeSH terms
Substances
Grants and funding
- 21ZXGWSY00100/Tianjin Science and Technology Major Special Project and Engineering Public Health Science and Technology Major Special Project
- Z-2017-26-1902-5/China International Medical Foundation
- ZXY-ZDSYSZD-1/Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital, Tianjin Key Laboratory of Metabolic Diseases
- 22JCZDJC00590/Key Project of Natural Science Foundation of Tianjin
- TJYXZDXK-032A/Tianjin Key Medical Discipline (Specialty) Construction Project
LinkOut - more resources
Full Text Sources
Medical
