Profiling Pro-Inflammatory Proteases as Biomolecular Signatures of Material-Induced Subcutaneous Host Response in Immuno-Competent Mice
- PMID: 39630111
- PMCID: PMC11792001
- DOI: 10.1002/advs.202309709
Profiling Pro-Inflammatory Proteases as Biomolecular Signatures of Material-Induced Subcutaneous Host Response in Immuno-Competent Mice
Abstract
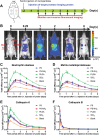
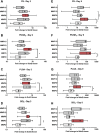
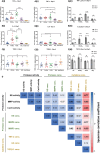
Proteases are important modulators of inflammation, but they remain understudied in material-induced immune response, which is critical to clinical success of biomedical implants. Herein, molecular expression and proteolytic activity of three distinct proteases, namely neutrophil elastase, matrix metalloproteinases, cysteine cathepsins (cathepsin-K and cathepsin-B) are comprehensively profiled, in the subcutaneous host response of immuno-competent mice against different biomaterial implants. Quantitative non-invasive monitoring with activatable fluorescent probes reveals that different microparticulate materials induce distinct levels of protease activity with degradable poly(lactic-co-glycolic) acid inducing the strongest signal compared to nondegradable materials such as polystyrene and silica oxide. Furthermore, protein expression of selected proteases, attributable to both their inactive and active forms, notably deviates from their activities associated only with their active forms. Protease activity exhibits positive correlations with protein expression of pro-inflammatory cytokines tumor necrosis factor α and interleukin 6 but negative correlation with pro-fibrotic cytokine transforming growth factor β1. This study also demonstrates the predictive utility of protease activity as a non-invasive, pro-inflammatory parameter for evaluation of the anti-inflammatory effects of model bioactive compounds on material-induced host response. Overall, the findings provide new insights into protease presence in material-induced immune responses, facilitating future biomaterial assessment to evoke appropriate host responses for implant applications.
Keywords: biomaterial; host immune response; inflammation; protease; subcutaneous implant.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhang D., Chen Q., Shi C., Chen M., Ma K., Wan J., Liu R., Adv. Funct. Mater. 2021, 31, 2007226.
-
- Anderson J., Cramer S., in Host Response to Biomaterials, (Ed: Badylak S. F.), Academic Press, Oxford: 2015, pp. 13–36.
-
- Jones J. A., McNally A. K., Chang D. T., Qin L. A., Meyerson H., Colton E., I. L. K. Kwon, T. Matsuda, J. M. Anderson, Japan. Soc. Biomater. Austral. Soc. Biomater. Korean Soc. Biomater. 2008, 84, 158.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
