The application and prospects of antimicrobial peptides in antiviral therapy
- PMID: 39630161
- PMCID: PMC11618130
- DOI: 10.1007/s00726-024-03427-0
The application and prospects of antimicrobial peptides in antiviral therapy
Abstract
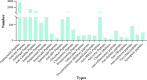
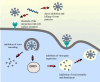
Antimicrobial peptides (AMPs) have broad-spectrum antimicrobial activity, enabling them to rapidly detect and eliminate targets. In addition, many AMPs are natural peptides, making them promising candidates for therapeutic drugs. This review discusses the basic properties and mechanisms of action of AMPs, highlighting their ability to disrupt microbial membranes and modulate host immune responses. It also reviews the current state of research into using AMPs against various viral infections, focusing on their therapeutic potential against viruses that contribute to the global health crisis. Despite promising developments, therapies based on AMPs still face challenges such as stability, toxicity, and production costs. In this text, we will discuss these challenges and the latest technological advances aimed at overcoming them. The combination of nanotechnology and bioengineering approaches offers new ways to enhance the delivery, efficacy, and safety of AMPs. We emphasize the importance of further research to fully exploit the potential of AMPs in antiviral therapy, advocating a multifaceted approach that includes optimizing clinical use and exploring synergies with existing antiviral drugs.
Keywords: Antimicrobial peptides (AMPs); Antiviral therapy; Coronaviruses; HIV; Influenza.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Aloul KM, Nielsen JE, Defensor EB, Lin JS, Fortkort JA, Shamloo M, Cirillo JD, Gombart AF, Barron AE (2022) Upregulating Human Cathelicidin Antimicrobial peptide LL-37 expression may prevent severe COVID-19 inflammatory responses and reduce microthrombosis. Front Immunol 13:880961. 10.3389/fimmu.2022.880961 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

