Unveiling the Antimycobacterial Potential of Novel 4-Alkoxyquinolines: Insights into Selectivity, Mechanism of Action, and In Vivo Exposure
- PMID: 39630172
- PMCID: PMC11684019
- DOI: 10.1021/acs.jmedchem.4c01302
Unveiling the Antimycobacterial Potential of Novel 4-Alkoxyquinolines: Insights into Selectivity, Mechanism of Action, and In Vivo Exposure
Abstract
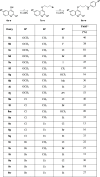
This work presents a comprehensive investigation into the design, synthesis, and evaluation of a novel series of 4-alkoxyquinolines as potential antimycobacterial agents. The design approach, which combined molecular simplification and chain extension, resulted in compounds with potent and selective activity against both drug-susceptible and multidrug-resistant Mycobacterium tuberculosis strains. The lead molecule, targeting the cytochrome bc1 complex, exhibited favorable kinetic solubility and remarkable chemical stability under acidic conditions. Despite in vitro ADME evaluations showing low permeability and high metabolism in rat microsomes, the lead compound exhibited bacteriostatic activity in a murine macrophage model of TB infection and demonstrated promising in vivo exposure following gavage in mice, with an AUC0-t of 127.5 ± 5.7 μM h. To the best of our knowledge, for the first time, a simplified structure from 2-(quinolin-4-yloxy)acetamides has shown such potential. These findings suggest a new avenue for exploring this chemical class as a source of antituberculosis drug candidates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- World Health Organization Global tuberculosis report 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa.... (Accessed 20 November 2023).
-
- WHO . WHO operational handbook on tuberculosis. Module 4: Treatment - Drug-Resistant Tuberculosis Treatment, 2022 update; World Health Organization: Geneva, 2022. https://www.who.int/publications/i/item/9789240065116. - PubMed
-
- Diacon A. H.; Pym A.; Grobusch M.; Patientia R.; Rustomjee R.; Page-Shipp L.; Pistorius C.; Krause R.; Bogoshi M.; Churchyard G.; Venter A.; Allen J.; Palomino J. C.; De Marez T.; van Heeswijk R. P.; Lounis N.; Meyvisch P.; Verbeeck J.; Parys W.; de Beule K.; Andries K.; Mc Neeley D. F. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009, 360 (23), 2397–2405. 10.1056/NEJMoa0808427. - DOI - PubMed
-
- Gler M. T.; Skripconoka V.; Sanchez-Garavito E.; Xiao H.; Cabrera-Rivero J. L.; Vargas-Vasquez D. E.; Gao M.; Awad M.; Park S. K.; Shim T. S.; Suh G. Y.; Danilovits M.; Ogata H.; Kurve A.; Chang J.; Suzuki K.; Tupasi T.; Koh W. J.; Seaworth B.; Geiter L. J.; Wells C. D. Delamanid for multidrug-resistant pulmonary tuberculosis. New England journal of medicine 2012, 366 (23), 2151–2160. 10.1056/NEJMoa1112433. - DOI - PubMed
-
- Conradie F.; Diacon A. H.; Ngubane N.; Howell P.; Everitt D.; Crook A. M.; Mendel C. M.; Egizi E.; Moreira J.; Timm J.; McHugh T. D.; Wills G. H.; Bateson A.; Hunt R.; Van Niekerk C.; Li M.; Olugbosi M.; Spigelman M.; Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382 (10), 893–902. 10.1056/NEJMoa1901814. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

