Burden of Myasthenia Gravis in the Czech Republic: Analysis of the Nationwide Patient Registry
- PMID: 39630385
- PMCID: PMC11762035
- DOI: 10.1007/s40120-024-00682-x
Burden of Myasthenia Gravis in the Czech Republic: Analysis of the Nationwide Patient Registry
Abstract
Introduction: The main goal of this study was to describe the Czech population of patients with MG in terms of demographics, disease characteristics, management approaches, and treatment trends.
Methods: We selected all patients, both incident and prevalent, who were enrolled in the Czech MyReg registry between August 24, 2015 and November 19, 2021. For the descriptive analysis, all patients enrolled in the registry, regardless of their date of diagnosis or date of enrolment, were included. We analyzed the following disease-related endpoints: myasthenia gravis composite (MGC) score, forced vital capacity (FVC), and Myasthenia Gravis Foundation of America (MGFA) clinical classification.
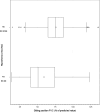
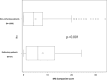
Results: The incidence showed a consistent increasing trend from 0.62 to 3.13. The mean MGC score was 5.0 (median 4.0, 95% CI 4.7, 5.3) representing mild form of MG. The difference in FVC from the predicted value in patients during and without myasthenic crisis was 58.93% (95% CI 37.27, 80.59) and 75.93% (95% CI 74.87, 77.00), respectively. We identified 70 patients (5.0%) with refractory MG, of whom 58.6% were female. The MGFA classifications in those with refractory vs. non-refractory disease was as follows: IIa 21.8% vs 23.2%, IIb 45.3% vs 33.6%, and IIIb 14.1% vs 4.6%, respectively.
Conclusion: Our analysis shows that the incidence of MG is increasing in the Czech Republic and that patients with refractory disease, of whom up to 58% are female, have a higher burden of disease than non-refractory patients.
Keywords: Burden of disease; FVC; MGC score; MGQoL15; Myasthenia gravis; Refractory patients.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Petr Hájek and Jacek Cudny are employees of UCB Pharma. Katarína Breciková and Aleš Tichopád are employees of CEEOR company, which was contracted by UCB Pharma to conduct the analyses. Stanislav Voháňka, Magda Horáková, Jana Junkerová, Michala Jakubíková, Jiří Piťha, Michaela Týblová and Daniela Vlažná claim they have no conflict of interest. The technical and analytical management of the register is provided by the Institute of Biostatistics and Analyses, s.r.o., which is also the processor of personal data within the register. CEEOR s.r.o. and IBA s.r.o. are commercial research organisations conducting observational studies. The following study centers participated on collection of data used in this work: University Hospital Brno—Department of Neurology, University Hospital Prague- Motol—Department of Child Neurology, University Hospital Ostrava—Department of Child Neurology, University Hospital Prague- Motol—Department of Neurology of the 2. Med. Faculty, University Hospital Ostrava—Department of Child Neurology, University Hospital Ostrava—Department of Neurology, University Hospital Plzeň—Department of Neurology, Thomayer University Hospital Prague—Department of Neurology, University Hospital Hradec Králové—Department of Neurology, University Hospital Prague- Královské Vinohrady—Department of Neurology, Hospital of the Pardubice region—Department of Neurology, Masaryk’s hospital in Ústí nad Labem—Neurology department, Hospital České Budějovice—Neurology department, Hospital in Teplice—Neurology department, General University Hospital Prague—Department of Neurology. University Hospital Brno and University Hospital Prague- Motol are members of the European Reference Network for Neuromuscular Diseases- Project ID 870177. Ethical Approval: The study was approved by the Ethics Committee of the University Hospital in Brno under the number 06-160518. All participants signed an informed consent. We confirm that this study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All authors had permission to use MyReg register.
Figures


References
-
- Verschuuren JJGM, Huijbers MG, Plomp JJ, Niks EH, Molenaar PC, Martinez-Martinez P, Gomez AM, de Baets MH, Losen M. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev. 2013. 10.1016/j.autrev.2013.03.001. - PubMed
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015. 10.1016/S1474-4422(15)00145-3. - PubMed
-
- Heldal A, Owe J, Gilhus N, Romi F. Seropositive myasthenia gravis: a nationwide epidemiologic study. Neurology. 2009;73:150–1. 10.1212/WNL.0b013e3181ad53c2. - PubMed
-
- Deenen J, Horlings C, Verschuuren J, Verbeek A, van Engelen B. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. 2015;2:73–85. - PubMed
LinkOut - more resources
Full Text Sources

