Distinct structural and functional connectivity of genetically segregated thalamoreticular subnetworks
- PMID: 39630580
- PMCID: PMC11922087
- DOI: 10.1016/j.celrep.2024.115037
Distinct structural and functional connectivity of genetically segregated thalamoreticular subnetworks
Abstract
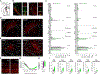
The thalamic reticular nucleus (TRN), the major inhibitory source of the thalamus, plays essential roles in sensory processing, attention, and cognition. However, our understanding of how TRN circuitry contributes to these diverse functions remains limited, largely due to the lack of genetic tools for selectively targeting TRN neurons with discrete structural and physiological properties. Here, we develop Cre mouse lines targeting two genetically segregated populations of TRN neurons that engage first-order (FO) and higher-order (HO) thalamic nuclei, respectively. In addition to substantially distinct electrophysiological properties, these TRN subnetworks are further distinguished by biases in top-down cortical and bottom-up thalamic inputs, along with significant differences in brain-wide synaptic convergence. Furthermore, we demonstrate that dysfunction of each subnetwork results in distinct cortical electroencephalogram (EEG) and sensory processing deficits commonly observed in neuropsychiatric disorders, underscoring the potential involvement of TRN subnetworks in the pathophysiology of these conditions.
Keywords: CP: Neuroscience; EEG; TRN subnetwork synaptic connectivity; electrophysiology; neurodevelopmental disorders; neuropsychiatric disorders; thalamic reticular nucleus, TRN; thalamocortical circuits.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

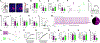
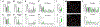

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

