YAP1 induces bladder cancer progression and promotes immune evasion through IL-6/STAT3 pathway and CXCL deregulation
- PMID: 39630608
- PMCID: PMC11735109
- DOI: 10.1172/JCI171164
YAP1 induces bladder cancer progression and promotes immune evasion through IL-6/STAT3 pathway and CXCL deregulation
Abstract
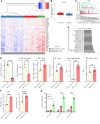
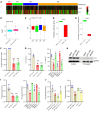
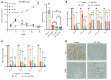
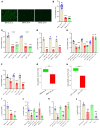
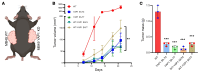
The Hippo signaling pathway plays a key role in tumorigenesis in different cancer types. We investigated the role of the Hippo effector YAP1 in the tumor immune microenvironment (TIME) of urothelial carcinoma of the bladder (UCB) and evaluated the efficacy of immunotherapy in the context of YAP1 signaling. We performed numerous in vitro and in vivo experiments to determine the role of YAP1 using genetic and pharmacological attenuation of YAP1 activity. Briefly, RNA sequencing was carried out with mouse and human cell lines to identify novel YAP1-regulated downstream targets unbiasedly. We then experimentally confirmed that YAP1 regulates the TIME through the IL-6/STAT3 signaling pathway and varied C-X-C motif chemokine regulation. We analyzed several human sample sets to explore the TIME status in the context of YAP1 expression. Our data indicate that YAP1 attenuation decreases M2 macrophages and myeloid-derived suppressor cells in the TIME compared with YAP1-expressing cells. In summary, this study provides insights into YAP1 signaling as a driver for cancer stemness and an inducer of immunosuppressive TIME. Moreover, the therapeutic efficacy of YAP1 attenuation indicates that combined blockade of YAP1 and immune checkpoints may yield clinical value for treating patients with UCB.
Keywords: Cancer immunotherapy; Oncogenes; Oncology; Therapeutics; Tumor suppressors.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

