Breast cancer cells promote osteoclast differentiation in an MRTF-dependent paracrine manner
- PMID: 39630611
- PMCID: PMC11742114
- DOI: 10.1091/mbc.E24-06-0285
Breast cancer cells promote osteoclast differentiation in an MRTF-dependent paracrine manner
Abstract
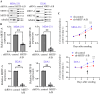
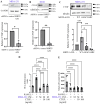
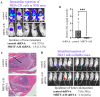
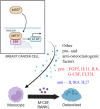
Bone is a frequent site for breast cancer metastasis. The vast majority of breast cancer-associated metastasis is osteolytic in nature, and RANKL (receptor activator for nuclear factor κB)-induced differentiation of bone marrow-derived macrophages to osteoclasts (OCLs) is a key requirement for osteolytic metastatic growth of cancer cells. In this study, we demonstrate that Myocardin-related transcription factor (MRTF) in breast cancer cells plays an important role in paracrine modulation of RANKL-induced OCL differentiation. This is partly attributed to MRTFs' critical role in maintaining the basal cellular expression of connective tissue growth factor (CTGF), findings that align with a strong positive correlation between CTGF expression and MRTF-A gene signature in the human disease context. Luminex analyses reveal that MRTF depletion in breast cancer cells has a broad impact on OCL-regulatory cell-secreted factors that extend beyond CTGF. Experimental metastasis studies demonstrate that MRTF depletion diminishes OCL abundance and bone colonization of breast cancer cells in vivo, suggesting that MRTF inhibition could be an effective strategy to diminish OCL formation and skeletal involvement in breast cancer. In summary, this study highlights a novel tumor-extrinsic function of MRTF relevant to breast cancer metastasis.
Figures




Update of
-
Breast cancer cells promote osteoclast differentiation in an MRTF-dependent paracrine manner.bioRxiv [Preprint]. 2024 Nov 7:2023.12.06.570453. doi: 10.1101/2023.12.06.570453. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Jan 1;36(1):ar8. doi: 10.1091/mbc.E24-06-0285. PMID: 38106226 Free PMC article. Updated. Preprint.
References
-
- Asparuhova MB, Ferralli J, Chiquet M, Chiquet-Ehrismann R (2011). The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J 25, 3477–3488. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

