Adjunctive Midodrine Therapy for Vasopressor-Dependent Shock in the ICU: A Systematic Review and Meta-Analysis
- PMID: 39631091
- PMCID: PMC11801447
- DOI: 10.1097/CCM.0000000000006519
Adjunctive Midodrine Therapy for Vasopressor-Dependent Shock in the ICU: A Systematic Review and Meta-Analysis
Abstract
Objectives: To summarize the efficacy of midodrine as an adjunctive therapy in critically ill patients. Safety of midodrine was assessed as a secondary outcome.
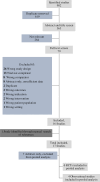
Data sources: We performed a systematic review and meta-analysis using a peer-reviewed search strategy combining the themes of vasopressor-dependent shock, critical care, and midodrine and including MEDLINE, Ovid Embase, CINAHL, and Cochrane library databases until September 14, 2023.
Study selection: We included studies if they: 1) included patients with vasopressor-dependent shock, 2) were performed in the ICU, 3) evaluated oral midodrine therapy compared with placebo or usual care, and 4) evaluated one of the outcomes of interest.
Data extraction: We extracted data independently in duplicate using standardized data abstraction forms, which included the following specific variables: patient characteristics, age, sex, type of ICU, etiology of shock, number of patients, study inclusion and exclusion criteria, and geographical location. We also captured the type, dose, and duration of IV vasopressors, any cointervention used, and outcome data.
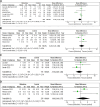
Data synthesis: We identified seven randomized controlled trials (six included in the pooled analysis) and ten observational studies (four included in the pooled analysis) that met eligibility criteria. Adjunctive midodrine may decrease ICU length of stay (LOS) and there is low certainty of effect on hospital LOS. Midodrine may decrease IV vasopressor support duration, ICU mortality, and hospital mortality. Pooled observational data was based on very low certainty data for all outcomes of interest. The trial sequential analysis-informed required sample size was not met for ICU LOS or IV vasopressor duration and this contributed to Grading of Recommendations, Assessment, Development, and Evaluations assessments of imprecision for both outcomes.
Conclusions: Adjunctive midodrine may decrease ICU LOS, duration of IV vasopressor therapy, and mortality in critically ill patients. However, required sample sizes was not met to determine our outcomes of interest. Midodrine may increase risk of bradycardia. While midodrine may provide benefit for patient-centered outcomes, due to increased risk of adverse events, further large-scale studies are needed to inform and guide its routine use in the ICU.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Mr. Kilcommons and Dr. Rewa disclosed the off-label product use of midodrine used for orthostatic hypotension and intradialytic hypotension. Dr. Bagshaw received funding from Baxter, Novartis, Sea Star Medical, BioPorto, and bioMerieux. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


References
-
- Vincent J, De Backer D: Circulatory shock. N Engl J Med. 2013; 369:1726–1734 - PubMed
-
- Levy MM, Evans LE, Rhodes A: The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018; 44:925–928 - PubMed
-
- Jentzer JC, Coons JC, Link CB, et al. : Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther. 2015; 20:249–260 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

