Adjuvant rituximab and elevated intratumoural CD8 expression are associated with sustained disease control after radiotherapy in a randomised trial of systemic therapy in early-stage follicular lymphoma
- PMID: 39631145
- PMCID: PMC11663783
- DOI: 10.1016/j.ebiom.2024.105468
Adjuvant rituximab and elevated intratumoural CD8 expression are associated with sustained disease control after radiotherapy in a randomised trial of systemic therapy in early-stage follicular lymphoma
Abstract
Background: We report extended follow-up of TROG99.03, a randomised phase III trial in early-stage follicular lymphoma (ESFL) including new information on the role of adjuvant rituximab and translational studies.
Methods: Patients with ESFL were randomised to involved field radiotherapy (IFRT) or IFRT plus 6-cycles cyclophosphamide/vincristine/prednisolone (IFRT + CVP). From 2006 rituximab was added to IFRT + CVP (IFRT + R-CVP). Clinical and multi-omic parameters were evaluated. Findings were validated in two independent ESFL cohorts (99 and 60 patients respectively).
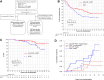
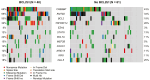
Findings: Between 2000 and 2012, 150 (75 per arm) patients were recruited. 48% were positron emission tomography (PET)-staged. By protocol, at median follow-up 11.3-years, progression-free survival (PFS) remained superior for IFRT+(R)CVP vs. IFRT (hazard ratio [HR] = 0.60, 95% CI = 0.37-0.98, p = 0.043; 10-year PFS 62% vs. 43%) respectively. Although no significant difference in overall survival was observed (HR = 0.44, 95% CI = 0.16-1.18, p = 0.11, 10-year OS 95% vs. 84%), patients receiving IFRT+(R)CVP experienced fewer composite (histological transformation and death) events (p = 0.045). PFS of IFRT + R-CVP-treated patients compared with all other treatments lacking rituximab (IFRT alone plus IFRT + CVP) was superior (HR = 0.36, 95% CI = 0.13-0.82, p = 0.013). Amongst PET-staged patients, PFS differences between IFRT + R-CVP vs. IFRT were maintained (HR = 0.38, 95% CI = 0.16-0.89, p = 0.027) indicating benefit distinct from stage migration. FL-related mutations and BCL2-translocations were not associated with PFS. However, by multivariate analysis elevated CD8A gene expression in diagnostic biopsy tissue was independently associated with improved PFS (HR = 0.45, 95% CI = 0.26-0.79, p = 0.037), a finding confirmed in both ESFL validation cohorts. CD8A gene expression was raised (p = 0.02) and CD8+ T-cell density higher within follicles in ESFL vs. advanced-stage FL (p = 0.047). Human leucocyte antigen class I specific neoantigens were detected in 43% of patients, suggesting neoantigen-specific CD8+ T-cells have a role in confining the spread of the disease.
Interpretation: Adjuvant R-CVP and elevated intratumoural CD8 expression were independently associated with sustained disease control after radiotherapy in ESFL.
Funding: Cancer Council Victora; National Health and Medical Research Council; Leukaemia Foundation; Mater Foundation.
Keywords: CD8; Early-stage follicular lymphoma; Neoantigen. randomized clinical trial; Radiotherapy; Rituximab.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Amgen (GCSF) and Roche (Rituximab) for the provision of study materials. There are no other relevant conflicts of interest.
Figures




References
-
- Teras L.R., DeSantis C.E., Cerhan J.R., Morton L.M., Jemal A., Flowers C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. - PubMed
-
- van Leeuwen M.T., Turner J.J., Joske D.J., et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982-2006. Int J Cancer. 2014;135(9):2146–2156. - PubMed
-
- Wirth A., Mikhaeel N.G., Aleman B.M.P., et al. Involved site radiation therapy in adult lymphomas: an overview of international lymphoma radiation Oncology group guidelines. Int J Radiat Oncol Biol Phys. 2020;107(5):909–933. - PubMed
-
- Marcus R., Davies A., Ando K., et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

