Host protein PRPS2 interact with the non-structural protein p17 of Avian Reovirus and promote viral replication
- PMID: 39631276
- PMCID: PMC11665346
- DOI: 10.1016/j.psj.2024.104582
Host protein PRPS2 interact with the non-structural protein p17 of Avian Reovirus and promote viral replication
Abstract
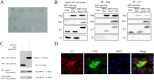
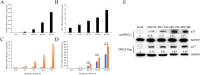
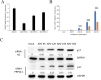
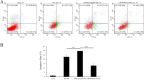
Avian reovirus (ARV) is highly prevalent in healthy poultry flocks and has been linked to viral arthritis/tendonitis, dwarf syndrome, chronic respiratory disease, and immunosuppression in avian species, resulting in significant economic losses within the poultry industry. The non-structural protein p17 encoded by ARV induces cellular autophagy and facilitates viral proliferation, playing a pivotal role in viral pathogenesis. To further elucidate the pathogenic mechanism basis of ARV p17 protein function, we employed a yeast two-hybrid system to identify Phosphoribosyl pyrophosphate synthetase 2 (PRPS2) as an interacting host protein with p17. In this study, we validated the interaction between PRPS2 and p17 using laser confocal microscopy, coimmunoprecipitation, and GST-Pulldown assays. Moreover, our findings demonstrate that the C-terminal region of PRPS2 is responsible for its binding to the p17 protein. Intriguingly, ARV infection significantly upregulated PRPS2 expression levels. Additionally, PRPS2 was shown to have a substantial impact on ARV replication; overexpression of PRPS2 increased ARV replication while knockdown of PRPS2 resulted in decreased quantities of ARV particles. Furthermore, our findings suggest that this process involves cellular apoptosis as a potential mechanism underlying these observations. Overall, this research provides valuable insights into elucidating the function of the p17 protein and sheds light on the pathogenic mechanism involving ARV-induced cellular apoptosis while offering novel perspectives for exploring therapeutic targets against ARV.
Keywords: Avian reovirus; PRPS2; Replication; p17 protein.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study. All authors have read the manuscript and approved to submit to poultry science.
Figures

References
-
- Abtin A., Shoushtari A., Fallah Mehrabadi M.H., Molouki A., Pourbakhsh S.A., Pourtaghi H., Eshratabadi F. Characterisation, whole-genome sequencing and phylogenetic analysis of three H3N2 avian influenza viruses isolated from domestic ducks at live poultry markets of Iran, 2017: first report. Vet. Med. Sci. 2022;8:1594–1602. - PMC - PubMed
-
- Ahmed M., Taylor W., Smith P.R., Becker M.A. Accelerated transcription of PRPS1 in X-linked overactivity of normal human phosphoribosylpyrophosphate synthetase. J. Biol. Chem. 1999;274:7482–7488. - PubMed
-
- Burd C.G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science (1979) 1994;265:615–621. - PubMed
-
- Chao-Yu H., Jyun-Yi L., En-Ying Y., Tsai-Ling L., Hsiao-Wei W., Pei-Chien T., Tz-Chuen J., Lon-Fye L., Brent L N., Hung-Jen L. The oncolytic Avian reovirus p17 protein inhibits invadopodia formation in Murine melanoma cancer cells by suppressing the FAK/Src pathway and the formation of theTKs5/NCK1 complex. Viruses. 2024;16 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

