Anesthesia alters complexity of spontaneous and stimulus-related neuronal firing patterns in rat visual cortex
- PMID: 39631661
- PMCID: PMC12397990
- DOI: 10.1016/j.neuroscience.2024.11.076
Anesthesia alters complexity of spontaneous and stimulus-related neuronal firing patterns in rat visual cortex
Abstract
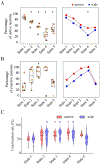
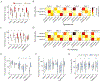
Complexity of neuronal firing patterns may serve as an indicator of sensory information processing across different states of consciousness. Recent studies have shown that spontaneous changes in brain states can occur during general anesthesia, which may influence neuronal complexity and the state of consciousness. In this study, we investigated how the firing patterns of cortical neurons, both at rest and during visual stimulation, are affected by spontaneously changing brain states under varying levels of anesthesia. Extracellular unit activity was measured in the primary visual cortex of unrestrained rats as the inhaled concentration of desflurane was incrementally reduced to 6%, 4%, 2%, and 0%. Using dimensionality reduction and density-based clustering on individual unit activities, we identified five distinct population states, which underwent dynamic transitions independent of the anesthetic level during both resting and stimulus conditions. One population state that occurred mainly in deep anesthesia exhibited a paradoxically increased number of active neurons and asynchronous spiking, suggesting a spontaneous reversal towards an awake-like condition. However, this was contradicted by the observation of low neuronal complexity in both spontaneous and stimulus-related spike activity, which more closely aligns with unconsciousness. Our findings reveal that transient neuronal states with distinct spiking patterns can emerge in visual cortex at constant anesthetic concentrations. The reduced complexity in states associated with deep anesthesia likely indicates a disruption of conscious sensory information processing.
Keywords: Anesthesia; Consciousness; Neuronal complexity; Spike dynamics; Visual cortex.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
State-Dependent Cortical Unit Activity Reflects Dynamic Brain State Transitions in Anesthesia.J Neurosci. 2020 Dec 2;40(49):9440-9454. doi: 10.1523/JNEUROSCI.0601-20.2020. Epub 2020 Oct 29. J Neurosci. 2020. PMID: 33122389 Free PMC article.
-
Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery.Cochrane Database Syst Rev. 2018 Aug 21;8(8):CD012317. doi: 10.1002/14651858.CD012317.pub2. Cochrane Database Syst Rev. 2018. PMID: 30129968 Free PMC article.
-
Effects of sevoflurane versus other general anaesthesia on emergence agitation in children.Cochrane Database Syst Rev. 2014 Sep 12;2014(9):CD007084. doi: 10.1002/14651858.CD007084.pub2. Cochrane Database Syst Rev. 2014. PMID: 25212274 Free PMC article.
References
-
- Altwegg-Boussac T, Schramm AE, Ballestero J, Grosselin F, Chavez M, Lecas S, Baulac M, Naccache L, et al. (2017), Cortical neurons and networks are dormant but fully responsive during isoelectric brain state. Brain 140:2381–2398. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

