Role of Myofibroblasts in the Repair of Iatrogenic Preterm Membranes Subjected to Mechanical Stimulation
- PMID: 39631799
- PMCID: PMC11717736
- DOI: 10.1002/pd.6722
Role of Myofibroblasts in the Repair of Iatrogenic Preterm Membranes Subjected to Mechanical Stimulation
Abstract
Objective: We examined the role of myofibroblasts in regulating Cx43 and collagen structure in iatrogenic preterm amniotic membrane (AM) defects subjected to mechanical stimulation.
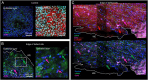
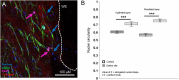
Method: Preterm AM specimens were collected from women undergoing planned preterm caesarean section after in utero intervention for correction of spina bifida by open fetal surgery (n = 4 patients; preterm delivery at 34 + 0 weeks to 35 + 0 weeks). Control specimens taken 5 cm away from the open fetal surgery defect site were compared with wound edge AM. In separate experiments, the effects of mechanical stimulation and co-treatment with Cx43 antisense on matrix and repair proteins were examined. Specimens were immunostained to detect αSMA and Cx43 in myofibroblasts and counterstained with DAPI to quantify nuclei shape. The direction of collagen fibrils in the wound edge region was examined by SHG imaging. Markers for matrix (collagen, elastin, GAG), inflammation (PGE2) and repair (TGFβ1) were examined by RT-qPCR and biochemical assays.
Results: In iatrogenic preterm AM specimens, the diameter of the open fetal surgery defect ranged between 3.5 and 7.5 cm. At the wound edge of the open fetal surgery defect, αSMA positive myofibroblasts had deformed nuclei and showed abundant Cx43 localized in the cell bodies or formed plaques. In the fibroblast layer, collagen had degenerated in some regions or had polarity near the wound edge. In preterm AM defects, mechanical stimulation and Cx43 antisense increased the levels of collagen and elastin but not GAG or PGE2 release. Mechanical stimulation increased Cx43 and TGFβ1 gene expression.
Conclusion: In open fetal surgery defects, myofibroblasts were elongated with collagen fibrils that either degenerated or had polarity. Whilst cells produced substantially higher Cx43 in the fibroblast than in the epithelial layer, they formed plaques, which may prevent migration and delay healing. Mechanical stimulation of preterm AM enhanced matrix repair proteins and the mechanotransduction should be explored to understand how Cx43 contributes to membrane integrity.
Keywords: Cx43; PPROM; fetal membranes; fetal surgery; iatrogenic; preterm birth.
© 2024 The Author(s). Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Devlieger D., Millar L. K., Bryant‐Greenwood G., Lewi L., and Deprest J., “Fetal Membrane Healing After Spontaneous and Iatrogenic Membrane Rupture: A Review of Current Evidence,” American Journal of Obstetrics and Gynecology 195, no. 6 (2006): 1512–1520, 10.1016/j.ajog.2006.01.074. - DOI - PMC - PubMed
-
- Joyce E. M., Moore J. J., and Sacks M. S., “Biomechanics of the Fetal Membrane Prior to Mechanical Failure: Review and Implications,” supplement, European Journal of Obstetrics & Gynecology and Reproductive Biology 144 Suppl 1, no. S1 (2009): S121–S127, 10.1016/j.ejogrb.2009.02.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

