Mitochondrial metabolic reprogramming of macrophages and T cells enhances CD47 antibody-engineered oncolytic virus antitumor immunity
- PMID: 39631851
- PMCID: PMC11624815
- DOI: 10.1136/jitc-2024-009768
Mitochondrial metabolic reprogramming of macrophages and T cells enhances CD47 antibody-engineered oncolytic virus antitumor immunity
Abstract
Background: Although immunotherapy can reinvigorate immune cells to clear tumors, the response rates are poor in some patients. Here, CD47 antibody-engineered oncolytic viruses (oAd-αCD47) were employed to lyse tumors and activate immunity. The oAd-αCD47 induced comprehensive remodeling of the tumor microenvironment (TME). However, whether the acidic TME affects the antitumor immunotherapeutic effects of oncolytic viruses-αCD47 has not been clarified.
Methods: To assess the impact of oAd-αCD47 treatment on the TME, we employed multicolor flow cytometry. Glucose uptake was quantified using 2NBDG, while mitochondrial content was evaluated with MitoTracker FM dye. pH imaging of tumors was performed using the pH-sensitive fluorophore SNARF-4F. Moreover, changes in the calmodulin-dependent protein kinase II (CaMKII)/cyclic AMP activates-responsive element-binding proteins (CREB) and peroxisome proliferator-activated receptor gamma coactivator-1α (PGC1α) signaling pathway were confirmed through western blotting and flow cytometry.
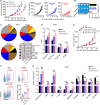
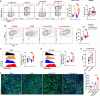
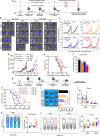
Results: Here, we identified sodium bicarbonate (NaBi) as the potent metabolic reprogramming agent that enhanced antitumor responses in the acidic TME. The combination of NaBi and oAd-αCD47 therapy significantly inhibited tumor growth and produced complete immune control in various tumor-bearing mouse models. Mechanistically, combination therapy mainly reduced the number of regulatory T cells and enriched the ratio of M1-type macrophages TAMs (M1.TAMs) to M2-type macrophages TAMs (M2.TAMs), while decreasing the abundance of PD-1+TIM3+ expression and increasing the expression of CD107a in the CD8+ T cells. Furthermore, the combination therapy enhanced the metabolic function of T cells and macrophages by upregulating PGC1α, a key regulator of mitochondrial biogenesis. This metabolic improvement contributed to a robust antitumor response. Notably, the combination therapy also promoted the generation of memory T cells, suggesting its potential as an effective neoadjuvant treatment for preventing postoperative tumor recurrence and metastasis.
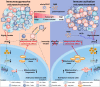
Conclusions: Tumor acidic microenvironment impairs mitochondrial energy metabolism in macrophages and T cells inducing oAd-αCD47 immunotherapeutic resistance. NaBi improves the acidity of the TME and activates the CaMKII/CREB/PGC1α mitochondrial biosynthesis signaling pathway, which reprograms the energy metabolism of macrophages and T cells in the TME, and oral NaBi enhances the antitumor effect of oAd-αCD47.
Keywords: Combination therapy; Immunosuppression; Immunotherapy; Oncolytic virus.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
