Comparative adherence and persistence of single-inhaler and multiple-inhaler triple therapies among patients with chronic obstructive pulmonary disease in Japan: a retrospective cohort study
- PMID: 39632104
- PMCID: PMC11624741
- DOI: 10.1136/bmjopen-2023-080864
Comparative adherence and persistence of single-inhaler and multiple-inhaler triple therapies among patients with chronic obstructive pulmonary disease in Japan: a retrospective cohort study
Abstract
Objectives: To evaluate and compare medication adherence and persistence for patients newly initiating single-inhaler triple therapy (SITT) and multiple-inhaler triple therapy (MITT) for chronic obstructive pulmonary disease (COPD) in Japan.
Design: Retrospective, new-user, active comparator, observational cohort study using inverse probability of treatment weighting.
Setting: Health insurance claims data from the Medical Data Vision Co., Ltd, hospital claims database.
Participants: Adults diagnosed with COPD at age ≥40 years newly initiating MITT or SITT (fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) or formoterol fumarate/budesonide/glycopyrronium) from 1 September 2019 to 31 July 2021.
Primary and secondary outcome measures: The primary outcome was medication adherence compared between patients using SITT and MITT, assessed by the proportion of days covered ≥80%. Secondary outcomes included medication persistence (time from index treatment initiation to discontinuation) compared between patients using SITT and MITT and medication adherence compared before and after the switch in a subgroup of patients switching from MITT to SITT.
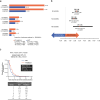
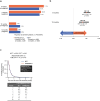
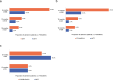
Results: We included 2575 MITT and 2962 SITT users with similar baseline characteristics following weighting. The proportion of adherent patients was significantly greater for SITT versus MITT users at 6 months (19.7% vs 10.2%, p<0.0001), 12 months (6.0% vs 3.8%, p=0.0009) and 18 months (3.8% vs 1.4%, p<0.0001) post-index. Median persistence was also significantly higher for SITT versus MITT users (2.0 vs 1.0 months, p<0.001). Comparing specific SITT versus MITT, the proportion of adherent patients at each time point and median persistence was greater for FF/UMEC/VI. In patients switching from MITT to SITT (n=688), the proportion of adherent patients increased postswitch at the class level and for FF/UMEC/VI specifically.
Conclusions: Patients with COPD in Japan who were newly initiating SITT had greater medication adherence and persistence compared with those on MITT up to 18 months following initiation.
Keywords: Drug Combinations; Medication Adherence; Medication Persistence; Pulmonary Disease, Chronic Obstructive; Respiratory Therapy.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SJ, AI, AM, CC, MY, KH and KJR are employees of and/or hold stocks/shares in GSK. AI is also an unpaid part-time professor at McMaster University, Hamilton, ON, Canada. RW, LC, OM, RS and TJ are employees of Adelphi Real World; Adelphi Real World received funds from GSK to complete the study. AC and TI were employees of GSK at the time of the study. Current affiliation details for AM and MY: Real World Data Analytics, Japan Development, GSK, Tokyo, Japan.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
