Early RSV infection aggravates asthma-related Th2 responses by increasing the number of CD4 + TRM cells through upregulation of PLZF
- PMID: 39632661
- PMCID: PMC12247139
- DOI: 10.3724/abbs.2024220
Early RSV infection aggravates asthma-related Th2 responses by increasing the number of CD4 + TRM cells through upregulation of PLZF
Abstract
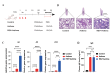
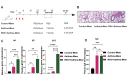
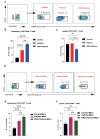
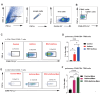
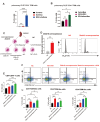
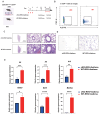
Respiratory syncytial virus (RSV) infection is correlated with the chronic pathogenesis and exacerbation of asthma. However, the mechanism remains unclear. In this study, acute and memory (Mem) asthma models with early RSV infection are established to explore the persistence of the effects of RSV infection on asthma. Intravascular injection of an anti-CD45 antibody is performed to define CD4 + TRM cells accurately. RSV infection has a sustained impact on asthma exacerbation for at least six weeks, with high Th2 cytokine secretion in lung tissue instead of IgE response-related B cells. CD45 -CD4 + TRM cells are positively correlated with RSV-related asthma exacerbation and severe airway inflammation. Mechanistically, overexpression of the transcription factor PLZF in vitro increases the number of CD4 + TRM cells, and conditional knockout of Zbtb16 (encoding PLZF) can decrease the number of CD4 + TRM cells to aggravate allergic inflammation and reduce Th2 responses. This study provides evidence for potential combined strategies that might benefit asthma patients.
Keywords: PLZF; asthma; respiratory syncytial virus infection; tissue-resident memory T cell.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
PLZF-expressing CD4+ T cells promote tissue-resident memory T cells in breaking immune tolerance in allergic asthma via IL-15/IL-15Rα signaling.Cell Commun Signal. 2025 Mar 15;23(1):138. doi: 10.1186/s12964-025-02134-x. Cell Commun Signal. 2025. PMID: 40089783 Free PMC article.
-
IFN-γ Attenuates Eosinophilic Inflammation but Is Not Essential for Protection against RSV-Enhanced Asthmatic Comorbidity in Adult Mice.Viruses. 2022 Jan 14;14(1):147. doi: 10.3390/v14010147. Viruses. 2022. PMID: 35062354 Free PMC article.
-
Host immune response to respiratory syncytial virus infection and its contribution to protection and susceptibility in adults: a systematic literature review.Expert Rev Clin Immunol. 2025 Jun;21(6):745-760. doi: 10.1080/1744666X.2025.2494658. Epub 2025 May 2. Expert Rev Clin Immunol. 2025. PMID: 40278893 Review.
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD009417. doi: 10.1002/14651858.CD009417.pub3. Cochrane Database Syst Rev. 2023. PMID: 37870128 Free PMC article.
-
Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses.Health Technol Assess. 2011 Jan;15(5):iii-iv, 1-124. doi: 10.3310/hta15050. Health Technol Assess. 2011. PMID: 21281564 Free PMC article.
References
-
- Dumas O, Erkkola R, Bergroth E, Hasegawa K, Mansbach JM, Piedra PA, Jartti T, et al. Severe bronchiolitis profiles and risk of asthma development in Finnish children. J Allergy Clin Immunol. . 2022;149:1281–1285.e1. doi: 10.1016/j.jaci.2021.08.035. - DOI - PubMed
-
- Tabor DE, Fernandes F, Langedijk AC, Wilkins D, Lebbink RJ, Tovchigrechko A, Ruzin A, et al. Global molecular epidemiology of respiratory syncytial virus from the 2017–2018 INFORM-RSV study. J Clin Microbiol. . 2020;59:e01828–20. doi: 10.1128/JCM.01828-20. - DOI - PMC - PubMed
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. . 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Cheng Q, He F, Zhao W, Xu X, Shang Y, Huang W. Histone acetylation regulates ORMDL3 expression-mediated NLRP3 inflammasome overexpression during RSV-allergic exacerbation mice. J Cell Physiol. . 2023;238:2904–2923. doi: 10.1002/jcp.31141. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

