Denfivontinib Activates Effector T Cells Through the NLRP3 Inflammasome, Yielding Potent Anticancer Effects by Combination with Pembrolizumab
- PMID: 39632711
- PMCID: PMC11876964
- DOI: 10.1158/1535-7163.MCT-24-0501
Denfivontinib Activates Effector T Cells Through the NLRP3 Inflammasome, Yielding Potent Anticancer Effects by Combination with Pembrolizumab
Abstract
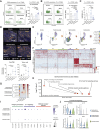
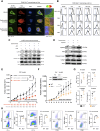
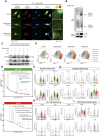
Various combination therapies have been investigated to overcome the limitations of using immune checkpoint inhibitors. However, determining the optimal combination therapy remains challenging. To overcome the therapeutic limitation, we conducted a translational research to elucidate the mechanisms by which AXL inhibition enhances antitumor effects when combined with anti-PD-1 antibody therapy. Herein, we demonstrated improved antitumor effects through combination treatment with denfivontinib and pembrolizumab which resulted in enhanced differentiation into effector CD4+ and CD8+ memory T cells, accompanied by an increase in IFN-γ expression in the YHIM-2004 xenograft model derived from patients with non-small cell lung cancer. Concurrently, a reduction in the number of immunosuppressive M2 macrophages and myeloid-derived suppressor cells was observed. Mechanistically, denfivontinib potentiated the NOD-like receptor pathway, thereby facilitating NLRP3 inflammasome formation. This leads to macrophage activation via NF-κB signaling pathway activation. We have confirmed that the positive interaction between macrophages and T cells arises from the enhanced antigen-presenting machinery of activated macrophages. Furthermore, the observed tumor effects in AXL knockout mice confirmed that AXL inhibition by denfivontinib enhances the antitumor effects, thus opening new avenues for therapeutic interventions aimed at overcoming limitations in immunotherapy. To demonstrate the extent to which our findings reflect clinical results, we analyzed bulk RNA sequencing data from 21 patients with non-small cell lung cancer undergoing anti-PD-1 immunotherapy. The NLRP3 inflammasome score influenced enhanced immune responses in patient data undergoing anti-PD-1 immunotherapy, suggesting a role for the NLRP3 inflammasome in activating immune responses during treatment.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
B.C. Cho reports personal fees from Abion, BeiGene, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squib, CJ, CureLogen, Cyrus Therapeutics, Ono, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI-Cell, Guardant, HK Inno-N, Imnewrun Biosciences Inc., Janssen, Takeda, MSD, Medpacto, Blueprint Medicines, RandBio, Hanmi, Yonsei University Health System, personal fees from Kanaph Therapeutics, BridgeBio Therapeutics, Cyrus Therapeutics, Guardant Health, Oscotec Inc, J INTS Bio, Therapex Co., Ltd, Gliead, Amgen, TheraCanVac Inc, Gencurix Inc, Interpark Bio Convergence Corp., Champions Oncology, Crown Bioscience, Imagen, and PearlRiver Bio GmbH, grants from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp, GI Innovation, GI-Cell, Abion, Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Onoclogy, CJ Bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, MSD, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, BridgeBio Therapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J INTS Bio, Hanmi, CHA Bundang Medical Center, and Vertical Bio AG, and other support from DAAN Biotherapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

