Bispecific antibodies targeting BCMA or GPRC5D are highly effective in relapsed myeloma after CAR T-cell therapy
- PMID: 39632797
- PMCID: PMC11618392
- DOI: 10.1038/s41408-024-01197-2
Bispecific antibodies targeting BCMA or GPRC5D are highly effective in relapsed myeloma after CAR T-cell therapy
Abstract
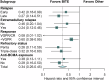
Despite the astonishing outcomes after chimeric antigen receptor (CAR) T-cell therapy for relapsed refractory multiple myeloma (RRMM), most patients eventually relapse. There are only limited data available on salvage therapies following relapse after BCMA-directed CAR T-cell therapy. Here, we analyzed outcomes of post-CAR T-cell therapy relapse and impact of different salvage strategies in an international cohort of 139 patients (n = 130 ide-cel, n = 9 cilta-cel), receiving talquetamab (n = 28), teclistamab (n = 37), combinations of immunomodulating drugs (IMiDs), proteasome inhibitors (PIs) or CD38 monoclonal antibodies (n = 43), and others (n = 31). The median time to relapse after CAR T-cell therapy was 5 months, 53% had the extramedullary disease (EMD) at relapse, associated with dismal post-relapse outcome (P = 0.005). Overall response and complete response upon salvage therapies were 79% and 39% for talquetamab, 64% and 32% for teclistamab, 30% and 0% for IMiDs/PIs/CD38, and 26% and 3% for others (P < 0.001). Duration of response, as well as median survival, was significantly improved with bispecific antibodies (P < 0.001, respectively). Bispecific antibodies seemed to overcome the poor prognosis associated with early relapse and EMD, and were independent predictors for improved survival in multivariable analysis. In summary, these results suggest bispecific antibodies as the standard of care for relapse after CAR T-cell therapy for RRMM.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Nico Gagelmann: Consulting or Advisory Role: Stemline Therapeutics, MorphoSys Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen. Maximilian Merz: Honoraria: Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Takeda, Sanofi, Pfizer Consulting or Advisory Role: Janssen, BMS GmbH & Co KG, Pfizer, Sanofi Research Funding: Janssen, SpringWorks Therapeutics, Roche/Genentech Travel, Accommodations, Expenses: Janssen, Stemline Therapeutics, Pfizer. Hamza Hashmi: Consulting or Advisory Role: Sanofi, Bristol Myers Squibb/Celgene, Janssen Speakers’ Bureau: Janssen, Karyopharm Therapeutics, Amgen. Nausheen Ahmed: Consulting or Advisory Role: Kite/Gilead, BMS Research Funding: Kite/Gilead (Inst). Aina Oliver-Caldés: Travel, Accommodations, Expenses: Janssen. Friedrich Stölzel: Honoraria: Medac, Jazz Pharmaceuticals, Consulting or Advisory Role: Glycostem, Travel, Accommodations, Expenses: SERVIER. Anca-Maria Albici: Honoraria: AbbVie, Travel, Accommodations, Expenses: SERVIER. Natalie Schub: Honoraria: Janssen Oncology, Consulting or Advisory Role: BMS, Travel, Accommodations, Expenses: Kite/Gilead. Soraya Kharboutli: Honoraria: Bristol Myers Squibb GmbH, Travel, Accommodations, Expenses: Janssen, Bristol Myers Squibb, Sobi, Novartis. Fabian Müller: Honoraria: AstraZeneca, Bristol Myers Squibb/Pfizer, Kite/Gilead, Consulting or Advisory Role: Bristol Myers Squibb/Pfizer, Janssen, Kite/Gilead, Kite/Gilead, Novartis, Miltenyi Biomedicine, Research Funding: Kite/Gilead, Travel, Accommodations, Expenses: SOBI, Janssen. Leyla Shune: Consulting or Advisory Role: Janssen Oncology. Faiz Anwer: Consulting or Advisory Role: Janssen, BMS, Speakers’ Bureau: Bristol Myers Squibb Foundation, Research Funding: Celgene (Inst), Acetylon Pharmaceuticals (Inst), Millennium (Inst), Astellas Pharma (Inst), AbbVie (Inst), Janssen (Inst), Bristol Myers Squibb (Inst), Caribou Biosciences (Inst), Caribou Biosciences, Travel, Accommodations, Expenses: Bristol Myers Squibb, Open Payments Link: https://openpaymentsdata.cms.gov/physician/16726 . Vladan Vucinic: Honoraria: Janssen, BMS GmbH & Co KG, Gilead Sciences, Amgen, Consulting or Advisory Role: Gilead Sciences, Janssen, BMS GmbH & Co KG, Amgen, Travel, Accommodations, Expenses: Sobi, Janssen, Gilead Sciences, Amgen. Uwe Platzbecker: Honoraria: Celgene/Jazz, AbbVie, Curis, Geron, Janssen, Consulting or Advisory Role: Celgene/Jazz, Novartis, BMS GmbH & Co KG, Research Funding: Amgen (Inst), Janssen (Inst), Novartis (Inst), BerGenBio (Inst), Celgene (Inst), Curis (Inst), Patents, Royalties, Other Intellectual Property: Part of a patent for a TFR-2 antibody (Rauner et al. Nature Metabolics 2019), Travel, Accommodations, Expenses: Celgene. Francis Ayuk: Honoraria: Bristol Myers Squibb/Celgene, Kite/Gilead, Janssen, Miltenyi Biomedicine, Novartis, Takeda, Mallinckrodt/Therakos, medac pharma, Consulting or Advisory Role: Bristol Myers Squibb/Celgene Research Funding: Mallinckrodt/Therakos. Nicolaus Kröger: Honoraria: Novartis, Celgene (Inst), Sanofi, Jazz Pharmaceuticals (Inst), Kite/Gilead, RIEMSER (Inst), AOP Orphan Pharmaceuticals, BMS GmbH & Co KG, Neovii, Alexion Pharmaceuticals, Takeda, Pierre Fabre Consulting or Advisory Role: Neovii, Sanofi, Jazz Pharmaceuticals, Novartis, Celgene, RIEMSER, Gilead Sciences, Speakers’ Bureau: AOP Orphan Pharmaceuticals, Research Funding: Neovii (Inst), Novartis (Inst), Celgene (Inst), Riemser (Inst), Travel, Accommodations, Expenses: Neovii, Novartis, Gilead Sciences, Jazz Pharmaceuticals, Sanofi, Celgene. Jack Khouri: Honoraria: GPCR Therapeutics, Consulting or Advisory Role: Janssen Oncology. Joseph McGuirk: Honoraria: Kite, a Gilead company, AlloVir, Magenta Therapeutics, Nektar, Sana Biotechnology Consulting or Advisory Role: Kite, a Gilead company, Juno Therapeutics, Allovir, Magenta Therapeutics, EcoR1 Capital, CRISPR therapeutics, Speakers’ Bureau: Kite/Gilead, Research Funding: Novartis (Inst), Fresenius Biotech (Inst), Astellas Pharma (Inst), Bellicum Pharmaceuticals (Inst), Novartis (Inst), Gamida Cell (Inst), Pluristem Therapeutics (Inst), Kite, a Gilead company (Inst), AlloVir (Inst), Travel, Accommodations, Expenses: Kite, a Gilead company, Syncopation Life Sciences, SITC/ACCC. Al-Ola Abdallah: Research Funding: Celgene (Inst), Seagen (Inst), AbbVie (Inst), Bristol Myers Squibb/Medarex (Inst), Sanofi (Inst), GlaxoSmithKline (Inst), Patents, Royalties, Other Intellectual Property: PSA vaccine patent. Carlos Fernández de Larrea: Honoraria: Janssen, BeiGene, Bristol Myers Squibb/Celgene, Pfizer, Amgen, GlaxoSmithKline Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Amgen, Pfizer, Sanofi, BeiGene Research Funding: Janssen (Inst), Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst) Travel, Accommodations, Expenses: Janssen, Amgen, GlaxoSmithKline, Bristol Myers Squibb/Celgene, BeiGene, Pfizer. No other potential conflicts of interest were reported.
Figures


References
-
- Lin Y, Qiu L, Usmani S, Joo CW, Costa L, Derman B et al. Consensus guidelines and recommendations for the management and response assessment of chimeric antigen receptor T-cell therapy in clinical practice for relapsed and refractory multiple myeloma: a report from the International Myeloma Working Group Immunotherapy Committee. Lancet Oncol 2024. e-pub ahead of print 20240528; 10.1016/S1470-2045(24)00094-9 - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. 10.1016/S0140-6736(21)00933-8. e-pub ahead of print 20210624 - DOI - PubMed
-
- Hansen DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience From the Myeloma CAR T Consortium. J Clin Oncol. 2023;41:2087–97. 10.1200/JCO.22.01365. e-pub ahead of print 20230109 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

