Role of intermittent hypoxic training combined with methazolamide in the prevention of high-altitude cerebral edema in rats
- PMID: 39632926
- PMCID: PMC11618614
- DOI: 10.1038/s41598-024-81226-z
Role of intermittent hypoxic training combined with methazolamide in the prevention of high-altitude cerebral edema in rats
Abstract
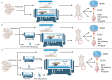
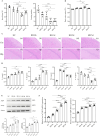
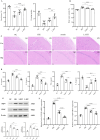
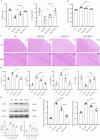
Although intermittent hypoxia training (IHT) and methazolamide (MTZ) alone can prevent high-altitude cerebral edema (HACE) to varying degrees, their efficacy and dispersion remain limited. However, only a handful of trials have explored the effectiveness of the IHT and MTZ combination in preventing HACE. Rats were first exposed to hypobaric hypoxia (5000 m, 54.02 kPa, 10.8% fraction of inspired oxygen (FiO2)) with simultaneous exhaustive exercise (EE) for different durations to determine the ideal condition for establishing a rat model of HACE. Rats receiving various courses of IHT were subjected to this condition, and changes in behaviour, brain water content (BWC), pathology and brain protein expression were evaluated. Meanwhile, rats received different doses of MTZ before and during hypoxia exposure with simultaneous EE. Finally, rats receiving the IHT and MTZ combination were then exposed to hypoxia with simultaneous EE. Systemic inflammation and mild cerebral edema developed in rats after 6 h of hypobaric hypoxia with simultaneous EE. Rats showed severe impairment of spatial and memory functions after 2 days of hypobaric hypoxia with simultaneous EE, and the pathology of their brain showed significant dilated perivascular spaces, cell swelling, vacuolar degeneration and reduced neuron count. BWC, serum inflammatory factors and expression of vascular endothelial growth factor (VEGF) and aquaporin 4 (AQP4) proteins in the hippocampus increased significantly. Both IHT and MTZ differentially counteracted hypobaric hypoxia-induced spatial and memory function impairments and increased BWC, pathological changes and expression of AQP4 and VEGF proteins in the hippocampus. Among these, the long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) showed the most significant improvement, restoring the rats' indices to normal levels. Continuous hypobaric hypoxia with simultaneous EE for 2 days resulted in significant HACE in rats, which may be used to establish a rat model of HACE. Both IHT and MTZ alleviated HACE in rats to varying degrees, among which long-course IHT (BID, 14 d) combined with MTZ (200 mg/kg/d) effectively prevented HACE in rats.
Keywords: Acute mountain sickness; High-altitude cerebral edema; Hypobaric hypoxia; Intermittent hypoxia training; Methazolamide.
© 2024. The Author(s).
Conflict of interest statement
Declaration. Competing interests: The authors declare no competing interests. Ethics approval: This study was approved by the Institutional Review Board of Beijing Shijitan Hospital, Capital Medical University, Approval No: sjtkyll-lx-2023(010). This study is reported in accordance with ARRIVE guidelines.
Figures
Similar articles
-
[Effects of Inhibiting the NKCC1/AQP4 Pathway on Neurological Injury Improvement in a Rat Model of High-Altitude Cerebral Edema].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Jan 20;56(1):156-165. doi: 10.12182/20250160204. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40109454 Free PMC article. Chinese.
-
Establishment of an experimental rat model of high altitude cerebral edema by hypobaric hypoxia combined with temperature fluctuation.Brain Res Bull. 2020 Dec;165:253-262. doi: 10.1016/j.brainresbull.2020.10.017. Epub 2020 Oct 24. Brain Res Bull. 2020. PMID: 33141074
-
High-altitude-induced cerebral edema in mice is alleviated by bestatin-mediated blood-brain barrier protection.J Neurophysiol. 2025 Jun 1;133(6):1902-1915. doi: 10.1152/jn.00381.2024. Epub 2025 May 13. J Neurophysiol. 2025. PMID: 40359176
-
Oxygen metabolism abnormalities and high-altitude cerebral edema.Front Immunol. 2025 Mar 19;16:1555910. doi: 10.3389/fimmu.2025.1555910. eCollection 2025. Front Immunol. 2025. PMID: 40176814 Free PMC article. Review.
-
Possible Role of Glymphatic System of the Brain in the Pathogenesis of High-Altitude Cerebral Edema.High Alt Med Biol. 2018 Dec;19(4):394-397. doi: 10.1089/ham.2018.0066. Epub 2018 Sep 21. High Alt Med Biol. 2018. PMID: 30239222 Review.
References
-
- Richalet, J.-P., Larmignat, P., Poitrine, E., Letournel, M. & Canouï-Poitrine, F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med185, 192–198. 10.1164/rccm.201108-1396OC (2012). - PubMed
-
- Gallagher, S. A. & Hackett, P. H. High-altitude illness. Emerg Med Clin North Am22 (2004). - PubMed
-
- Wilson, M. H., Newman, S. & Imray, C. H. The cerebral effects of ascent to high altitudes. Lancet Neurol8, 175–191. 10.1016/S1474-4422(09)70014-6 (2009). - PubMed
-
- Bärtsch, P. & Swenson, E. R. Clinical practice: Acute high-altitude illnesses. N Engl J Med368, 2294–2302. 10.1056/NEJMcp1214870 (2013). - PubMed
-
- Netzer, N., Strohl, K., Faulhaber, M., Gatterer, H. & Burtscher, M. Hypoxia-related altitude illnesses. J Travel Med20, 247–255. 10.1111/jtm.12017 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

