Sex differences in the association of dietary inflammatory index with chronic kidney disease in US adults
- PMID: 39632932
- PMCID: PMC11618389
- DOI: 10.1038/s41598-024-78307-4
Sex differences in the association of dietary inflammatory index with chronic kidney disease in US adults
Abstract
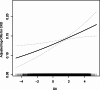
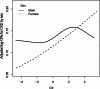
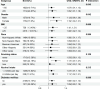
Studies on the association between dietary inflammatory index (DII) and chronic kidney disease (CKD) are limited. We aimed to examine the association between DII and CKD among U.S. adults with particular attention paid to sex differences. A total of 19317participants were included in this study. The exposure variable was DII, which was calculated based on overall inflammatory effect scores. The outcome was CKD, defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or urinary albumin/creatinine (uACR) ≥ 30 mg/g. The mean (SD) of age our study participants was 47.84 (18.35); and the mean (SD) of DII was 1.50 (1.91) (median 1.74). In multivariate logistic regression analysis, we observed that the OR value (95%CI) of CKD is 1.19 (1.14, 1.23), 1.12 (1.06, 1.19), and 1.16 (1.06, 1.27) in models 1, 2 and 3 for Per SD increment of DII. Compared with Participants with Q1(DII < 0.15), the adjusted ORs for participants in Q2 (0.15 ≤ DII < 1.74), Q3 (1.74 ≤ DII < 3.02) and Q4 (≥ 3.02)were 1.46 (95% CI 1.14-1.88), 1.55 (95% CI 1.20-1.99) and 1.52 (95% CI 1.17, 1.98) ( p for trend < 0.05), respectively. However, this study observed that the independent positive correlation between DII and CKD appeared in women rather than men. Higher DII levels were significantly and linearly associated with an increased prevalence of chronic kidney disease, and sex modified the association. This suggests that gender-specific dietary interventions can be developed to reduce the risk of CKD.
Keywords: Chronic kidney disease; Dietary inflammatory index; Females; Sex differences.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: All procedures performed in studies involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided written informed consent. And this survey was approved by the Ethics Review Board of the National Center for Health Statistics. Informed consent: Informed consent was obtained from all individual participants included in the study.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

