X-chromosome-wide association study for Alzheimer's disease
Affiliations
- 1 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, LabEx DISTALZ - U1167-RID-AGE Facteurs de Risque et Déterminants Moléculaires des Maladies Liées au Vieillissement, Lille, France.
- 2 The John P. Hussman Institute for Human Genomics, University of Miami, Miami, FL, USA.
- 3 Institute of Biomedicine, University of Eastern Finland, Kuopio, Finland.
- 4 Nuffield Department of Population Health Oxford University, Oxford, UK.
- 5 Department of Epidemiology, Erasmus MC, Rotterdam, The Netherlands.
- 6 Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
- 7 Department of Medicine, Cardiovascular Health Research Unit, University of Washington, Seattle, WA, USA.
- 8 Department of Old Age Psychiatry and Cognitive Disorders, University Hospital Bonn, University of Bonn, Bonn, Germany.
- 9 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany.
- 10 Division of Neurogenetics and Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University of Cologne, Medical Faculty, Cologne, Germany.
- 11 Department of Public Health Sciences, University of Miami Miller School of Medicine, Miami, FL, USA.
- 12 Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
- 13 Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Amsterdam Neuroscience, Vrije University, Amsterdam, The Netherlands.
- 14 Research Center and Memory Clinic, ACE Alzheimer Center Barcelona, Universitat Internacional de Catalunya, Barcelona, Spain.
- 15 CIBERNED, Network Center for Biomedical Research in Neurodegenerative Diseases, National Institute of Health Carlos III, Madrid, Spain.
- 16 Department of Neurology, Albert Einstein College of Medicine, New York, NY, USA.
- 17 Neurogenomics Division, Translational Genomics Research Institute, Phoenix, AZ, USA.
- 18 Arizona Alzheimer's Consortium, Phoenix, AZ, USA.
- 19 Banner Alzheimer's Institute, Phoenix, AZ, USA.
- 20 Department of Psychiatry, University of Arizona, Phoenix, AZ, USA.
- 21 Department of Neurology, Boston University, Boston, MA, USA.
- 22 Department of Pathology, Boston University, Boston, MA, USA.
- 23 Program in Translational Neuro-Psychiatric Genomics, Institute for the Neurosciences, Department of Neurology & Psychiatry, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
- 24 Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA.
- 25 Department of Epidemiology and Biostatistics, Case Western Reserve University, Cleveland, OH, USA.
- 26 Taub Institute on Alzheimer's Disease and the Aging Brain, Department of Neurology, Columbia University, New York, NY, USA.
- 27 Department of Neurology, Columbia University, New York, NY, USA.
- 28 Department of Neurology, Emory University, Atlanta, GA, USA.
- 29 Department of Radiology, Indiana University, Indianapolis, IN, USA.
- 30 Department of Medical and Molecular Genetics, Indiana University, Indianapolis, IN, USA.
- 31 Department of Neurology, Johns Hopkins University, Baltimore, MD, USA.
- 32 Department of Neurology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
- 33 Department of Neurology, Mayo Clinic, Rochester, MN, USA.
- 34 Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA.
- 35 Department of Psychiatry, Mount Sinai School of Medicine, New York, NY, USA.
- 36 Center for Cognitive Neurology and Departments of Neurology, New York University, School of Medicine, New York, NY, USA.
- 37 Department of Psychiatry, New York University, New York, NY, USA.
- 38 Cognitive Neurology and Alzheimer's Disease Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 39 Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 40 Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, USA.
- 41 Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA.
- 42 Department of Pathology (Neuropathology), Rush University Medical Center, Chicago, IL, USA.
- 43 Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA.
- 44 Department of Neurology & Neurological Sciences, Stanford University, Stanford, CA, USA.
- 45 Department of Neurology, University of Alabama at Birmingham, Birmingham, AL, USA.
- 46 Department of Neurology, University of California Davis, Sacramento, CA, USA.
- 47 Department of Neurobiology and Behavior, University of California Irvine, Irvine, CA, USA.
- 48 Department of Neurosciences, University of California San Diego, La Jolla, CA, USA.
- 49 University of Kansas Alzheimer's Disease Center, University of Kansas Medical Center, Kansas City, KS, USA.
- 50 Sanders-Brown Center on Aging and University of Kentucky Alzheimer's Disease Research Center, Department of Neuroscience, University of Kentucky, Lexington, KY, USA.
- 51 Michigan Alzheimer's Disease Center, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
- 52 Penn Neurodegeneration Genomics Center, Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
- 53 University of Pittsburgh Alzheimer's Disease Research Center, Pittsburgh, PA, USA.
- 54 Department of Neurology, University of Southern California, Los Angeles, CA, USA.
- 55 Department of Medicine, University of Washington, Seattle, WA, USA.
- 56 Department of Neurology, University of Washington, Seattle, WA, USA.
- 57 Department of Radiology, University of Washington, Seattle, WA, USA.
- 58 Department of Epidemiology, University of Washington, Seattle, WA, USA.
- 59 Geriatric Research, Education and Clinical Center (GRECC), University of Wisconsin, Madison, WI, USA.
- 60 Department of Medicine, University of Wisconsin, Madison, WI, USA.
- 61 Wisconsin Alzheimer's Disease Research Center, Madison, WI, USA.
- 62 Gerontology and Geriatric Medicine Center on Diabetes, Obesity, and Metabolism, Wake Forest School of Medicine, Winston-Salem, NC, USA.
- 63 Program in Cellular Neuroscience, Neurodegeneration & Repair, Yale University, New Haven, CT, USA.
- 64 Department of Psychiatry and Hope Center Program on Protein Aggregation and Neurodegeneration, Washington University School of Medicine, St. Louis, MO, USA.
- 65 Cleveland Clinic Lou Ruvo Center for Brain Health, Cleveland Clinic, Cleveland, OH, USA.
- 66 Department of Neuroscience, Mount Sinai School of Medicine, New York, NY, USA.
- 67 Department of Human Genetics, University of Pittsburgh, Pittsburgh, PA, USA.
- 68 Department of Medicine (Neurology), Tanz Centre for Research in Neurodegenerative Disease, Temerty Faculty of Medicine, University of Toronto, and University Health Network, Toronto, ON, USA.
- 69 Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Department of Neurology, Columbia University Irving Medical Center, 630 West 168th Street, New York, NY, 10032, USA.
- 70 Klinik für Psychiatrie und Psychotherapie, Saarbrücken, Germany.
- 71 Department of Neurology, Washington University, St. Louis, MO, USA.
- 72 Department of Pathology and Immunology, Washington University, St. Louis, MO, USA.
- 73 Estudios en Neurociencias y Sistemas Complejos (ENyS) CONICET-HEC-UNAJ, Buenos Aires, Argentina.
- 74 Division of Psychological Medicine and Clinical Neuroscience, School of Medicine, Cardiff University, Wales, UK.
- 75 Complex Genetics of Alzheimer's Disease Group, VIB Center for Molecular Neurology, VIB, Antwerp, Belgium.
- 76 Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
- 77 German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany.
- 78 Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Psychiatry and Psychotherapy, Hindenburgdamm 30, 12203, Berlin, Germany.
- 79 Department for Neurodegenerative Diseases and Geriatric Psychiatry, University Hospital Bonn, Venusberg-Campus 1, 53127, Bonn, Germany.
- 80 Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Munich, Germany.
- 81 German Center for Neurodegenerative Diseases (DZNE), Munich, Germany.
- 82 Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
- 83 Martin-Luther-University Halle-Wittenberg, University Clinic and Outpatient Clinic for Psychiatry, Psychotherapy and Psychosomatics, Halle (Saale), Germany.
- 84 Department of Psychiatry and Psychotherapy, LVR-Klinikum Essen, University of Duisburg-Essen, Germany, Medical Faculty, Duisburg, Germany.
- 85 Department of Psychiatry, Psychosomatics and Psychotherapy, Center of Mental Health, University Hospital of Würzburg, Würzburg, Germany.
- 86 Institute of Social Medicine, Occupational Health and Public Health, University of Leipzig, 04103, Leipzig, Germany.
- 87 Department of Geriatric Psychiatry, Central Institute for Mental Health Mannheim, Faculty Mannheim, University of Heidelberg, Heidelberg, Germany.
- 88 Alzheimer's Disease and Other Cognitive Disorders Unit, Neurology Service, Hospital Clínic of Barcelona, Fundació Recerca Clinic Barcelona- Institut d'Investigacions Biomèdiques August Pi i Sunyer (FRCB-IDIBAPS), and University of Barcelona, Barcelona, Spain.
- 89 Neurological Tissue Bank-Biobank, Hospital Clinic-FRCB-IDIBAPS, Barcelona, Spain.
- 90 German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany.
- 91 Institute of Cognitive Neurology and Dementia Research (IKND), Otto-von-Guericke University, Magdeburg, Germany.
- 92 Center for Cognitive Disorders, Department of Psychiatry and Psychotherapy, Technical University of Munich, School of Medicine and Health, Klinikum rechts der Isar, Munich, Germany.
- 93 Department of Psychiatry and Psychotherapy, University Medical Center Goettingen, Goettingen, Germany.
- 94 German Center for Neurodegenerative Diseases (DZNE), Goettingen, Germany.
- 95 Medical Science Department, iBiMED, Aveiro, Portugal.
- 96 Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany.
- 97 Institute for Urban Public Health, University Hospital of University Duisburg-Essen, Essen, Germany.
- 98 1st Department of Neurology, Medical school, Aristotle University of Thessaloniki, Thessaloniki, Makedonia, Greece.
- 99 Taub Institute for Research in Alzheimer's Disease and the Aging Brain, The Gertrude H. Sergievsky Center, Depatment of Neurology, Columbia University, New York, NY, USA.
- 100 1st Department of Neurology, Aiginition Hospital, National and Kapodistrian University of Athens, Medical School, Athens, Greece.
- 101 Sant Pau Memory Unit, Institut de Recerca Sant Pau (IR Sant Pau), Department of Neurology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
- 102 Department of Neurology, Hospital Universitario Donostia, San Sebastian, Spain.
- 103 Neurosciences Area, Instituto Biodonostia, San Sebastian, Spain.
- 104 Unitat de Genètica Molecular, Institut de Biomedicina de València-CSIC, Valencia, Spain.
- 105 Unidad Mixta de Neurologia Genètica, Instituto de Investigación Sanitaria La Fe, Valencia, Spain.
- 106 Centro de Biología Molecular Severo Ochoa (UAM-CSIC), Madrid, Spain.
- 107 Instituto de Investigacion Sanitaria 'Hospital la Paz' (IdIPaz), Madrid, Spain.
- 108 Universidad Autónoma de Madrid, Madrid, Spain.
- 109 Fundació Docència i Recerca MútuaTerrassa, Terrassa, Barcelona, Spain.
- 110 Memory Disorders Unit, Department of Neurology, Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain.
- 111 Alzheimer's Disease and Other Cognitive Disorders Unit, Service of Neurology, Hospital Clínic of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona, Spain.
- 112 Laboratorio de Genética, Hospital Universitario Central de Asturias, Oviedo, Spain.
- 113 Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Spain.
- 114 Unidad de Trastornos del Movimiento, Servicio de Neurología y Neurofisiología, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío/CSIC/Universidad de Sevilla, Seville, Spain.
- 115 Unidad Clínica de Enfermedades Infecciosas y Microbiología, Hospital Universitario de Valme, Sevilla, Spain.
- 116 Depatamento de Especialidades Quirúrgicas, Bioquímica e Inmunología, Facultad de Medicina, Universidad de Málaga, Málaga, Spain.
- 117 Unitat Trastorns Cognitius, Hospital Universitari Santa Maria de Lleida, Lleida, Spain.
- 118 Institut de Recerca Biomedica de Lleida (IRBLLeida), Lleida, Spain.
- 119 Alzheimer Research Center & Memory Clinic, Andalusian Institute for Neuroscience, Málaga, Spain.
- 120 Neurology Service, Marqués de Valdecilla University Hospital (University of Cantabria and IDIVAL), Santander, Spain.
- 121 Institute of Clinical Medicine-Neurology, University of Eastern Finland, Kuopio, Finland.
- 122 Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
- 123 Clinic of Neurology, UH "Alexandrovska", Medical University-Sofia, Sofia, Bulgaria.
- 124 Memory Clinic, Department of Neurology, Charles University, Second Faculty of Medicine and Motol University Hospital, Praha, Czech Republic.
- 125 Department of Clinical Biochemistry, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark.
- 126 Department of Clinical Genetics, VU University Medical Centre, Amsterdam, The Netherlands.
- 127 Department of Neurology, Erasmus MC, Rotterdam, The Netherlands.
- 128 Maastricht University, Department of Psychiatry & Neuropsychologie, Alzheimer Center Limburg, Maastricht, The Netherlands.
- 129 Unit for Hereditary Dementias, Theme Aging, Karolinska University Hospital-Solna, 171 64, Stockholm, Sweden.
- 130 Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
- 131 Department of Public Health and Caring Sciences/Geriatrics, Uppsala University, Uppsala, Sweden.
- 132 Université Paris-Saclay, CEA, Centre National de Recherche en Génomique Humaine, 91057, Evry, France.
- 133 Univ Rouen Normandie, Normandie Univ, Inserm U1245 and CHU Rouen, Department of Genetics and CNRMAJ, F-76000, Rouen, France.
- 134 Inserm, Bordeaux Population Health Research Center, UMR 1219, Univ. Bordeaux, ISPED, CIC 1401-EC, Univ. Bordeaux, Bordeaux, France.
- 135 CHU de Bordeaux, Pole Santé Publique, Bordeaux, France.
- 136 Univ. Lille, Inserm 1171, CHU Clinical and Research Memory Research Centre (CMRR) of Distalz, Lille, France.
- 137 Université de Paris, EA 4468, APHP, Hôpital Broca, Paris, France.
- 138 University Bordeaux, Inserm, Bordeaux Population Health Research Center, Bordeaux, France.
- 139 Department of Neurology, Bordeaux University Hospital, Bordeaux, France.
- 140 Department of Child and Adolescent Psychiatry and Psychotherapy, University Hospital of Psychiatry Zurich, University of Zurich, Zurich, Switzerland.
- 141 Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland.
- 142 Zurich Center for Integrative Human Physiology, University of Zurich, Zurich, Switzerland.
- 143 Old Age Psychiatry, Department of Psychiatry, Lausanne University Hospital, Lausanne, Switzerland.
- 144 Department of Geriatric Psychiatry, University Hospital of Psychiatry Zürich, Zürich, Switzerland.
- 145 Institute for Regenerative Medicine, University of Zürich, Zurich, Switzerland.
- 146 Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, 25125, Italy.
- 147 Neurodegenerative Diseases Unit, Fondazione IRCCS Ca' Granda, Ospedale Policlinico, Milan, Italy.
- 148 Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
- 149 Department of Clinical Sciences and Community Health, University of Milan, 20122, Milan, Italy.
- 150 Geriatric Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, 20122, Milan, Italy.
- 151 Institute of Gerontology and Geriatrics, Department of Medicine and Surgery, University of Perugia, Perugia, Italy.
- 152 Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden.
- 153 Interdisciplinary Department of Medicine, Geriatric Medicine and Memory Unit, University of Bari "A. Moro", Bari, Italy.
- 154 Centre for Memory Disturbances, Lab of Clinical Neurochemistry, Section of Neurology, University of Perugia, Perugia, Italy.
- 155 Department of Biomedical Sciences, Section of Neuroscience and Clinical Pharmacology, University of Cagliari, Cagliari, Italy.
- 156 Neurology Unit, "San Gerardo" Hospital, Monza and University of Milano-Bicocca, Milan, Italy.
- 157 Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
- 158 Cognitive and Behavioural Neurology, Department of Continuity of Care and Frailty, ASST Spedali Civili Brescia, Brescia, Italy.
- 159 Department of Neuroscience, Psychology, Drug Research and Child Health University of Florence, Florence, Italy.
- 160 IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
- 161 Department of Medicine and Surgery, Unit of Neurology, University-Hospital of Parma, Parma, Italy.
- 162 Department of Hematology and Stem Cell Transplant, Vito Fazzi Hospital, Lecce, Italy.
- 163 Department of Neuroscience "Rita Levi Montalcini", University of Torino, Torino, Italy.
- 164 Department of Neuroscience, Università Cattolica del Sacro Cuore, Rome, Italy.
- 165 Neurology Unit, IRCCS Fondazione Policlinico Universitario A. Gemelli, Rome, Italy.
- 166 Laboratory of Experimental Neuropsychobiology, IRCCS Santa Lucia Foundation, Rome, Italy.
- 167 Institute of Neurology, Catholic University of the Sacred Heart, Rome, Italy.
- 168 Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
- 169 Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
- 170 Cluster of Excellence Cellular Stress Responses in Aging-associated Diseases (CECAD), University of Cologne, Cologne, Germany.
- 171 Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
- 172 Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
- 173 Laboratory of Genetics, Immunology and Human Pathology, Faculty of Science of Tunis, University of Tunis El Manar, 2092, Tunis, Tunisia.
- 174 Alzheimer's Centre Reina Sofia-CIEN Foundation-ISCIII, Madrid, Spain.
- 175 Krembil Brain Institute, University Health Network, Toronto, ON, Canada.
- 176 Tanz Centre for Research in Neurodegenerative Diseases, Departments of Medicine and Laboratory Medicine & Pathobiology, University of Toronto, Toronto, ON, Canada.
- 177 Department of Population and Quantitative Health Sciences and Cleveland Institute for Computational Biology, Case Western Reserve University, Cleveland, OH, USA.
- 178 Department of Biostatistics, Boston University, Boston, MA, USA.
- 179 Department of Epidemiology, Boston University, Boston, MA, USA.
- 180 Department of Medicine (Biomedical Genetics), Boston University, Boston, MA, USA.
- 181 Department of Ophthalmology, Boston University, Boston, MA, USA.
- 182 Gertrude H. Sergievsky Center, Columbia University, New York, NY, USA.
- 183 Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
- 184 Glenn Biggs Institute for Alzheimer's and Neurodegenerative Diseases, San Antonio, TX, USA.
- 185 Boston University and the NHLBI's Framingham Heart Study, Boston, MA, USA.
- 186 Department of Neurology, Boston University School of Medicine, Boston, MA, USA.
- 187 UK Dementia Research Institute, Cardiff University, Cardiff, UK.
- 188 Department of Psychiatry & Glenn Biggs Institute for Alzheimer's and Neurodegenerative Diseases, San Antonio, TX, USA.
- 189 Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Disease (CECAD), University of Cologne, Cologne, Germany.
- 190 Dr. John T. Macdonald Foundation Department of Human Genetics, University of Miami, Miami, FL, USA.
- 191 NORMENT Centre, Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway.
- 192 Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
- 193 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, QC, Canada.
- 194 University of Miami Miller School of Medicine, Miami, FL, USA.
- 195 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, LabEx DISTALZ - U1167-RID-AGE Facteurs de Risque et Déterminants Moléculaires des Maladies Liées au Vieillissement, Lille, France. celine.bellenguez@pasteur-lille.fr.
- PMID: 39633006
- PMCID: PMC12092188
- DOI: 10.1038/s41380-024-02838-5
X-chromosome-wide association study for Alzheimer's disease
Authors
Affiliations
- 1 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, LabEx DISTALZ - U1167-RID-AGE Facteurs de Risque et Déterminants Moléculaires des Maladies Liées au Vieillissement, Lille, France.
- 2 The John P. Hussman Institute for Human Genomics, University of Miami, Miami, FL, USA.
- 3 Institute of Biomedicine, University of Eastern Finland, Kuopio, Finland.
- 4 Nuffield Department of Population Health Oxford University, Oxford, UK.
- 5 Department of Epidemiology, Erasmus MC, Rotterdam, The Netherlands.
- 6 Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
- 7 Department of Medicine, Cardiovascular Health Research Unit, University of Washington, Seattle, WA, USA.
- 8 Department of Old Age Psychiatry and Cognitive Disorders, University Hospital Bonn, University of Bonn, Bonn, Germany.
- 9 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany.
- 10 Division of Neurogenetics and Molecular Psychiatry, Department of Psychiatry and Psychotherapy, University of Cologne, Medical Faculty, Cologne, Germany.
- 11 Department of Public Health Sciences, University of Miami Miller School of Medicine, Miami, FL, USA.
- 12 Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
- 13 Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Amsterdam Neuroscience, Vrije University, Amsterdam, The Netherlands.
- 14 Research Center and Memory Clinic, ACE Alzheimer Center Barcelona, Universitat Internacional de Catalunya, Barcelona, Spain.
- 15 CIBERNED, Network Center for Biomedical Research in Neurodegenerative Diseases, National Institute of Health Carlos III, Madrid, Spain.
- 16 Department of Neurology, Albert Einstein College of Medicine, New York, NY, USA.
- 17 Neurogenomics Division, Translational Genomics Research Institute, Phoenix, AZ, USA.
- 18 Arizona Alzheimer's Consortium, Phoenix, AZ, USA.
- 19 Banner Alzheimer's Institute, Phoenix, AZ, USA.
- 20 Department of Psychiatry, University of Arizona, Phoenix, AZ, USA.
- 21 Department of Neurology, Boston University, Boston, MA, USA.
- 22 Department of Pathology, Boston University, Boston, MA, USA.
- 23 Program in Translational Neuro-Psychiatric Genomics, Institute for the Neurosciences, Department of Neurology & Psychiatry, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
- 24 Program in Medical and Population Genetics, Broad Institute, Cambridge, MA, USA.
- 25 Department of Epidemiology and Biostatistics, Case Western Reserve University, Cleveland, OH, USA.
- 26 Taub Institute on Alzheimer's Disease and the Aging Brain, Department of Neurology, Columbia University, New York, NY, USA.
- 27 Department of Neurology, Columbia University, New York, NY, USA.
- 28 Department of Neurology, Emory University, Atlanta, GA, USA.
- 29 Department of Radiology, Indiana University, Indianapolis, IN, USA.
- 30 Department of Medical and Molecular Genetics, Indiana University, Indianapolis, IN, USA.
- 31 Department of Neurology, Johns Hopkins University, Baltimore, MD, USA.
- 32 Department of Neurology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
- 33 Department of Neurology, Mayo Clinic, Rochester, MN, USA.
- 34 Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA.
- 35 Department of Psychiatry, Mount Sinai School of Medicine, New York, NY, USA.
- 36 Center for Cognitive Neurology and Departments of Neurology, New York University, School of Medicine, New York, NY, USA.
- 37 Department of Psychiatry, New York University, New York, NY, USA.
- 38 Cognitive Neurology and Alzheimer's Disease Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 39 Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 40 Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, USA.
- 41 Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, USA.
- 42 Department of Pathology (Neuropathology), Rush University Medical Center, Chicago, IL, USA.
- 43 Department of Epidemiology and Population Health, Stanford University, Stanford, CA, USA.
- 44 Department of Neurology & Neurological Sciences, Stanford University, Stanford, CA, USA.
- 45 Department of Neurology, University of Alabama at Birmingham, Birmingham, AL, USA.
- 46 Department of Neurology, University of California Davis, Sacramento, CA, USA.
- 47 Department of Neurobiology and Behavior, University of California Irvine, Irvine, CA, USA.
- 48 Department of Neurosciences, University of California San Diego, La Jolla, CA, USA.
- 49 University of Kansas Alzheimer's Disease Center, University of Kansas Medical Center, Kansas City, KS, USA.
- 50 Sanders-Brown Center on Aging and University of Kentucky Alzheimer's Disease Research Center, Department of Neuroscience, University of Kentucky, Lexington, KY, USA.
- 51 Michigan Alzheimer's Disease Center, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
- 52 Penn Neurodegeneration Genomics Center, Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
- 53 University of Pittsburgh Alzheimer's Disease Research Center, Pittsburgh, PA, USA.
- 54 Department of Neurology, University of Southern California, Los Angeles, CA, USA.
- 55 Department of Medicine, University of Washington, Seattle, WA, USA.
- 56 Department of Neurology, University of Washington, Seattle, WA, USA.
- 57 Department of Radiology, University of Washington, Seattle, WA, USA.
- 58 Department of Epidemiology, University of Washington, Seattle, WA, USA.
- 59 Geriatric Research, Education and Clinical Center (GRECC), University of Wisconsin, Madison, WI, USA.
- 60 Department of Medicine, University of Wisconsin, Madison, WI, USA.
- 61 Wisconsin Alzheimer's Disease Research Center, Madison, WI, USA.
- 62 Gerontology and Geriatric Medicine Center on Diabetes, Obesity, and Metabolism, Wake Forest School of Medicine, Winston-Salem, NC, USA.
- 63 Program in Cellular Neuroscience, Neurodegeneration & Repair, Yale University, New Haven, CT, USA.
- 64 Department of Psychiatry and Hope Center Program on Protein Aggregation and Neurodegeneration, Washington University School of Medicine, St. Louis, MO, USA.
- 65 Cleveland Clinic Lou Ruvo Center for Brain Health, Cleveland Clinic, Cleveland, OH, USA.
- 66 Department of Neuroscience, Mount Sinai School of Medicine, New York, NY, USA.
- 67 Department of Human Genetics, University of Pittsburgh, Pittsburgh, PA, USA.
- 68 Department of Medicine (Neurology), Tanz Centre for Research in Neurodegenerative Disease, Temerty Faculty of Medicine, University of Toronto, and University Health Network, Toronto, ON, USA.
- 69 Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Department of Neurology, Columbia University Irving Medical Center, 630 West 168th Street, New York, NY, 10032, USA.
- 70 Klinik für Psychiatrie und Psychotherapie, Saarbrücken, Germany.
- 71 Department of Neurology, Washington University, St. Louis, MO, USA.
- 72 Department of Pathology and Immunology, Washington University, St. Louis, MO, USA.
- 73 Estudios en Neurociencias y Sistemas Complejos (ENyS) CONICET-HEC-UNAJ, Buenos Aires, Argentina.
- 74 Division of Psychological Medicine and Clinical Neuroscience, School of Medicine, Cardiff University, Wales, UK.
- 75 Complex Genetics of Alzheimer's Disease Group, VIB Center for Molecular Neurology, VIB, Antwerp, Belgium.
- 76 Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
- 77 German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany.
- 78 Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Psychiatry and Psychotherapy, Hindenburgdamm 30, 12203, Berlin, Germany.
- 79 Department for Neurodegenerative Diseases and Geriatric Psychiatry, University Hospital Bonn, Venusberg-Campus 1, 53127, Bonn, Germany.
- 80 Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Munich, Germany.
- 81 German Center for Neurodegenerative Diseases (DZNE), Munich, Germany.
- 82 Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
- 83 Martin-Luther-University Halle-Wittenberg, University Clinic and Outpatient Clinic for Psychiatry, Psychotherapy and Psychosomatics, Halle (Saale), Germany.
- 84 Department of Psychiatry and Psychotherapy, LVR-Klinikum Essen, University of Duisburg-Essen, Germany, Medical Faculty, Duisburg, Germany.
- 85 Department of Psychiatry, Psychosomatics and Psychotherapy, Center of Mental Health, University Hospital of Würzburg, Würzburg, Germany.
- 86 Institute of Social Medicine, Occupational Health and Public Health, University of Leipzig, 04103, Leipzig, Germany.
- 87 Department of Geriatric Psychiatry, Central Institute for Mental Health Mannheim, Faculty Mannheim, University of Heidelberg, Heidelberg, Germany.
- 88 Alzheimer's Disease and Other Cognitive Disorders Unit, Neurology Service, Hospital Clínic of Barcelona, Fundació Recerca Clinic Barcelona- Institut d'Investigacions Biomèdiques August Pi i Sunyer (FRCB-IDIBAPS), and University of Barcelona, Barcelona, Spain.
- 89 Neurological Tissue Bank-Biobank, Hospital Clinic-FRCB-IDIBAPS, Barcelona, Spain.
- 90 German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany.
- 91 Institute of Cognitive Neurology and Dementia Research (IKND), Otto-von-Guericke University, Magdeburg, Germany.
- 92 Center for Cognitive Disorders, Department of Psychiatry and Psychotherapy, Technical University of Munich, School of Medicine and Health, Klinikum rechts der Isar, Munich, Germany.
- 93 Department of Psychiatry and Psychotherapy, University Medical Center Goettingen, Goettingen, Germany.
- 94 German Center for Neurodegenerative Diseases (DZNE), Goettingen, Germany.
- 95 Medical Science Department, iBiMED, Aveiro, Portugal.
- 96 Institute of Human Genetics, University of Bonn, School of Medicine & University Hospital Bonn, Bonn, Germany.
- 97 Institute for Urban Public Health, University Hospital of University Duisburg-Essen, Essen, Germany.
- 98 1st Department of Neurology, Medical school, Aristotle University of Thessaloniki, Thessaloniki, Makedonia, Greece.
- 99 Taub Institute for Research in Alzheimer's Disease and the Aging Brain, The Gertrude H. Sergievsky Center, Depatment of Neurology, Columbia University, New York, NY, USA.
- 100 1st Department of Neurology, Aiginition Hospital, National and Kapodistrian University of Athens, Medical School, Athens, Greece.
- 101 Sant Pau Memory Unit, Institut de Recerca Sant Pau (IR Sant Pau), Department of Neurology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
- 102 Department of Neurology, Hospital Universitario Donostia, San Sebastian, Spain.
- 103 Neurosciences Area, Instituto Biodonostia, San Sebastian, Spain.
- 104 Unitat de Genètica Molecular, Institut de Biomedicina de València-CSIC, Valencia, Spain.
- 105 Unidad Mixta de Neurologia Genètica, Instituto de Investigación Sanitaria La Fe, Valencia, Spain.
- 106 Centro de Biología Molecular Severo Ochoa (UAM-CSIC), Madrid, Spain.
- 107 Instituto de Investigacion Sanitaria 'Hospital la Paz' (IdIPaz), Madrid, Spain.
- 108 Universidad Autónoma de Madrid, Madrid, Spain.
- 109 Fundació Docència i Recerca MútuaTerrassa, Terrassa, Barcelona, Spain.
- 110 Memory Disorders Unit, Department of Neurology, Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain.
- 111 Alzheimer's Disease and Other Cognitive Disorders Unit, Service of Neurology, Hospital Clínic of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona, Spain.
- 112 Laboratorio de Genética, Hospital Universitario Central de Asturias, Oviedo, Spain.
- 113 Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Spain.
- 114 Unidad de Trastornos del Movimiento, Servicio de Neurología y Neurofisiología, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío/CSIC/Universidad de Sevilla, Seville, Spain.
- 115 Unidad Clínica de Enfermedades Infecciosas y Microbiología, Hospital Universitario de Valme, Sevilla, Spain.
- 116 Depatamento de Especialidades Quirúrgicas, Bioquímica e Inmunología, Facultad de Medicina, Universidad de Málaga, Málaga, Spain.
- 117 Unitat Trastorns Cognitius, Hospital Universitari Santa Maria de Lleida, Lleida, Spain.
- 118 Institut de Recerca Biomedica de Lleida (IRBLLeida), Lleida, Spain.
- 119 Alzheimer Research Center & Memory Clinic, Andalusian Institute for Neuroscience, Málaga, Spain.
- 120 Neurology Service, Marqués de Valdecilla University Hospital (University of Cantabria and IDIVAL), Santander, Spain.
- 121 Institute of Clinical Medicine-Neurology, University of Eastern Finland, Kuopio, Finland.
- 122 Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
- 123 Clinic of Neurology, UH "Alexandrovska", Medical University-Sofia, Sofia, Bulgaria.
- 124 Memory Clinic, Department of Neurology, Charles University, Second Faculty of Medicine and Motol University Hospital, Praha, Czech Republic.
- 125 Department of Clinical Biochemistry, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark.
- 126 Department of Clinical Genetics, VU University Medical Centre, Amsterdam, The Netherlands.
- 127 Department of Neurology, Erasmus MC, Rotterdam, The Netherlands.
- 128 Maastricht University, Department of Psychiatry & Neuropsychologie, Alzheimer Center Limburg, Maastricht, The Netherlands.
- 129 Unit for Hereditary Dementias, Theme Aging, Karolinska University Hospital-Solna, 171 64, Stockholm, Sweden.
- 130 Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
- 131 Department of Public Health and Caring Sciences/Geriatrics, Uppsala University, Uppsala, Sweden.
- 132 Université Paris-Saclay, CEA, Centre National de Recherche en Génomique Humaine, 91057, Evry, France.
- 133 Univ Rouen Normandie, Normandie Univ, Inserm U1245 and CHU Rouen, Department of Genetics and CNRMAJ, F-76000, Rouen, France.
- 134 Inserm, Bordeaux Population Health Research Center, UMR 1219, Univ. Bordeaux, ISPED, CIC 1401-EC, Univ. Bordeaux, Bordeaux, France.
- 135 CHU de Bordeaux, Pole Santé Publique, Bordeaux, France.
- 136 Univ. Lille, Inserm 1171, CHU Clinical and Research Memory Research Centre (CMRR) of Distalz, Lille, France.
- 137 Université de Paris, EA 4468, APHP, Hôpital Broca, Paris, France.
- 138 University Bordeaux, Inserm, Bordeaux Population Health Research Center, Bordeaux, France.
- 139 Department of Neurology, Bordeaux University Hospital, Bordeaux, France.
- 140 Department of Child and Adolescent Psychiatry and Psychotherapy, University Hospital of Psychiatry Zurich, University of Zurich, Zurich, Switzerland.
- 141 Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland.
- 142 Zurich Center for Integrative Human Physiology, University of Zurich, Zurich, Switzerland.
- 143 Old Age Psychiatry, Department of Psychiatry, Lausanne University Hospital, Lausanne, Switzerland.
- 144 Department of Geriatric Psychiatry, University Hospital of Psychiatry Zürich, Zürich, Switzerland.
- 145 Institute for Regenerative Medicine, University of Zürich, Zurich, Switzerland.
- 146 Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, 25125, Italy.
- 147 Neurodegenerative Diseases Unit, Fondazione IRCCS Ca' Granda, Ospedale Policlinico, Milan, Italy.
- 148 Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
- 149 Department of Clinical Sciences and Community Health, University of Milan, 20122, Milan, Italy.
- 150 Geriatric Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, 20122, Milan, Italy.
- 151 Institute of Gerontology and Geriatrics, Department of Medicine and Surgery, University of Perugia, Perugia, Italy.
- 152 Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden.
- 153 Interdisciplinary Department of Medicine, Geriatric Medicine and Memory Unit, University of Bari "A. Moro", Bari, Italy.
- 154 Centre for Memory Disturbances, Lab of Clinical Neurochemistry, Section of Neurology, University of Perugia, Perugia, Italy.
- 155 Department of Biomedical Sciences, Section of Neuroscience and Clinical Pharmacology, University of Cagliari, Cagliari, Italy.
- 156 Neurology Unit, "San Gerardo" Hospital, Monza and University of Milano-Bicocca, Milan, Italy.
- 157 Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
- 158 Cognitive and Behavioural Neurology, Department of Continuity of Care and Frailty, ASST Spedali Civili Brescia, Brescia, Italy.
- 159 Department of Neuroscience, Psychology, Drug Research and Child Health University of Florence, Florence, Italy.
- 160 IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
- 161 Department of Medicine and Surgery, Unit of Neurology, University-Hospital of Parma, Parma, Italy.
- 162 Department of Hematology and Stem Cell Transplant, Vito Fazzi Hospital, Lecce, Italy.
- 163 Department of Neuroscience "Rita Levi Montalcini", University of Torino, Torino, Italy.
- 164 Department of Neuroscience, Università Cattolica del Sacro Cuore, Rome, Italy.
- 165 Neurology Unit, IRCCS Fondazione Policlinico Universitario A. Gemelli, Rome, Italy.
- 166 Laboratory of Experimental Neuropsychobiology, IRCCS Santa Lucia Foundation, Rome, Italy.
- 167 Institute of Neurology, Catholic University of the Sacred Heart, Rome, Italy.
- 168 Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
- 169 Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
- 170 Cluster of Excellence Cellular Stress Responses in Aging-associated Diseases (CECAD), University of Cologne, Cologne, Germany.
- 171 Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
- 172 Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
- 173 Laboratory of Genetics, Immunology and Human Pathology, Faculty of Science of Tunis, University of Tunis El Manar, 2092, Tunis, Tunisia.
- 174 Alzheimer's Centre Reina Sofia-CIEN Foundation-ISCIII, Madrid, Spain.
- 175 Krembil Brain Institute, University Health Network, Toronto, ON, Canada.
- 176 Tanz Centre for Research in Neurodegenerative Diseases, Departments of Medicine and Laboratory Medicine & Pathobiology, University of Toronto, Toronto, ON, Canada.
- 177 Department of Population and Quantitative Health Sciences and Cleveland Institute for Computational Biology, Case Western Reserve University, Cleveland, OH, USA.
- 178 Department of Biostatistics, Boston University, Boston, MA, USA.
- 179 Department of Epidemiology, Boston University, Boston, MA, USA.
- 180 Department of Medicine (Biomedical Genetics), Boston University, Boston, MA, USA.
- 181 Department of Ophthalmology, Boston University, Boston, MA, USA.
- 182 Gertrude H. Sergievsky Center, Columbia University, New York, NY, USA.
- 183 Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
- 184 Glenn Biggs Institute for Alzheimer's and Neurodegenerative Diseases, San Antonio, TX, USA.
- 185 Boston University and the NHLBI's Framingham Heart Study, Boston, MA, USA.
- 186 Department of Neurology, Boston University School of Medicine, Boston, MA, USA.
- 187 UK Dementia Research Institute, Cardiff University, Cardiff, UK.
- 188 Department of Psychiatry & Glenn Biggs Institute for Alzheimer's and Neurodegenerative Diseases, San Antonio, TX, USA.
- 189 Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Disease (CECAD), University of Cologne, Cologne, Germany.
- 190 Dr. John T. Macdonald Foundation Department of Human Genetics, University of Miami, Miami, FL, USA.
- 191 NORMENT Centre, Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway.
- 192 Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
- 193 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, QC, Canada.
- 194 University of Miami Miller School of Medicine, Miami, FL, USA.
- 195 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, LabEx DISTALZ - U1167-RID-AGE Facteurs de Risque et Déterminants Moléculaires des Maladies Liées au Vieillissement, Lille, France. celine.bellenguez@pasteur-lille.fr.
- PMID: 39633006
- PMCID: PMC12092188
- DOI: 10.1038/s41380-024-02838-5
Abstract
Due to methodological reasons, the X-chromosome has not been featured in the major genome-wide association studies on Alzheimer's Disease (AD). To address this and better characterize the genetic landscape of AD, we performed an in-depth X-Chromosome-Wide Association Study (XWAS) in 115,841 AD cases or AD proxy cases, including 52,214 clinically-diagnosed AD cases, and 613,671 controls. We considered three approaches to account for the different X-chromosome inactivation (XCI) states in females, i.e. random XCI, skewed XCI, and escape XCI. We did not detect any genome-wide significant signals (P ≤ 5 × 10-8) but identified seven X-chromosome-wide significant loci (P ≤ 1.6 × 10-6). The index variants were common for the Xp22.32, FRMPD4, DMD and Xq25 loci, and rare for the WNK3, PJA1, and DACH2 loci. Overall, this well-powered XWAS found no genetic risk factors for AD on the non-pseudoautosomal region of the X-chromosome, but it identified suggestive signals warranting further investigations.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Written informed consent was obtained from study participants or, for those with substantial cognitive impairment, a caregiver, legal guardian or other proxy. Study protocols for all cohorts were reviewed and approved by the appropriate institutional review boards.
Figures
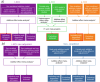
Fig. 1. Study design of the AD-XWAS.
Fig. 1. Study design of the AD-XWAS.
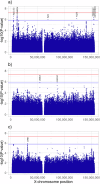
Fig. 2. Manhattan plot of the r-XCI…
Fig. 2. Manhattan plot of the r-XCI approach.
Association results of a the meta-analysis including…

Fig. 3. Manhattan plot of the e-XCI…
Fig. 3. Manhattan plot of the e-XCI approach.
Association results of a the diagnosed AD-cases…

Fig. 4. Manhattan plot of the s-XCI…
Fig. 4. Manhattan plot of the s-XCI approach meta-analysis, which excludes biobanks.
The red and…

