Choroidal vasculitis as a biomarker of inflammation of the choroid. Indocyanine Green Angiography (ICGA) spearheading for diagnosis and follow-up, an imaging tutorial
- PMID: 39633039
- PMCID: PMC11618284
- DOI: 10.1186/s12348-024-00442-w
Choroidal vasculitis as a biomarker of inflammation of the choroid. Indocyanine Green Angiography (ICGA) spearheading for diagnosis and follow-up, an imaging tutorial
Abstract
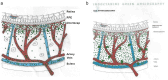
Background: Indocyanine green angiography (ICGA) is the gold standard to diagnose, evaluate and follow up choroidal inflammation. It allows clinicians to precisely determine the type and extension of choroidal vasculitis in the two main choroidal structures, the choriocapillaris and the choroidal stroma. The presence of choroidal vasculitis is often overlooked by the physician who often does not include ICGA in the investigation of posterior uveitis.
Purpose: To describe choroidal vasculitis by analysing its ICGA signs in order to investigate and follow choroiditis and determine the pathophysiological mechanisms of inflammation of choroidal vessels.
Methods: The tutorial is presenting the normal findings in a non-inflamed choroid and the semiology of diverse choroidal vasculitis conditions, followed by practical illustrations using typical cases.
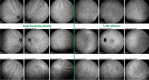
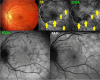
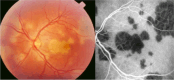
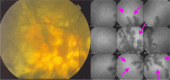
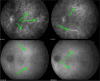
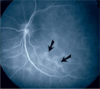
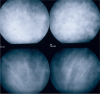
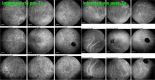
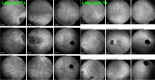
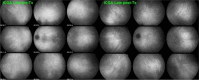
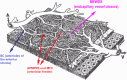
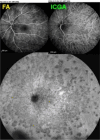
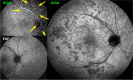
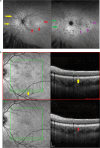
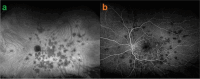
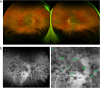
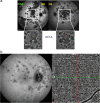
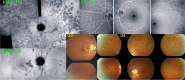
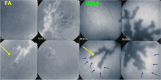
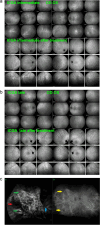
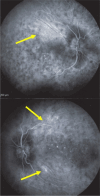
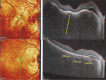
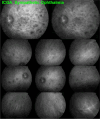
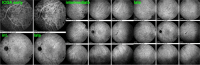
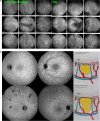
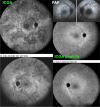
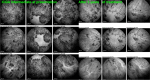
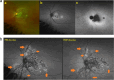
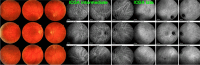
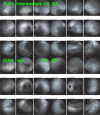
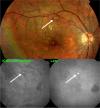
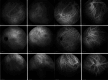
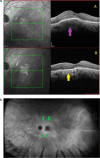
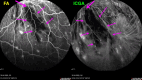
Results: The two identified patterns of choroidal vasculitis corresponded on one side to choriocapillaritis appearing as areas of hypofluorescence depicting the involvement and extension of choriocapillaris inflammatory non-perfusion. The vasculitis of the choriocapillaris goes from limited and reversible when distal endcapillary vessels are involved such as in Multiple Evanescent White Dot Syndrome (MEWDS) to more severe involvement in Acute Posterior Multifocal Placoid Pigment Epitheliopathy (APMPPE), Multifocal Choroiditis (MFC) or Serpiginous Choroiditis (SC) with more pronounced non-perfusion causing scars if not treated diligently. On the other side, stromal choroidal vasculitis is characterised by leaking hyperfluorescent vessels that appear fuzzy and at the origin of late diffuse choroidal hyperfluorescence.
Conclusion: Choroidal vasculitis is present in almost all patients with inflammatory choroidal involvement, occlusive in case of choriocapillaritis and leaky in stromal choroiditis causing vessel hyperfluorescence, fuzziness of the choroidal vessels and late diffuse stromal hyperfluorescence on ICGA. Systemic vasculitis entities produce occlusive vasculitis of large choroidal vessels.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Our ethical committee (EC-COSMTC) waived the need of approval due to retrospective study. Consent for publication: All participants have signed a consent form. Competing interests: Ioannis Papasavvas is a co-author of this review and associate editor of the journal. The rest of the authors have no competing interest to declare.
Figures




Similar articles
-
Assessment and classification of choroidal vasculitis in posterior uveitis using indocyanine green angiography.Klin Monbl Augenheilkd. 2002 Apr;219(4):243-9. doi: 10.1055/s-2002-30661. Klin Monbl Augenheilkd. 2002. PMID: 12022010
-
Classification of Non-Infectious and/or Immune Mediated Choroiditis: A Brief Overview of the Essentials.Diagnostics (Basel). 2021 May 24;11(6):939. doi: 10.3390/diagnostics11060939. Diagnostics (Basel). 2021. PMID: 34073914 Free PMC article. Review.
-
Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview.Medicina (Kaunas). 2022 Jan 21;58(2):165. doi: 10.3390/medicina58020165. Medicina (Kaunas). 2022. PMID: 35208488 Free PMC article. Review.
-
Diagnosis, Mechanisms, and Differentiation of Inflammatory Diseases of the Outer Retina: Photoreceptoritis versus Choriocapillaritis; A Multimodal Imaging Perspective.Diagnostics (Basel). 2022 Sep 9;12(9):2179. doi: 10.3390/diagnostics12092179. Diagnostics (Basel). 2022. PMID: 36140579 Free PMC article.
-
Mechanisms, Pathophysiology and Current Immunomodulatory/Immunosuppressive Therapy of Non-Infectious and/or Immune-Mediated Choroiditis.Pharmaceuticals (Basel). 2022 Mar 24;15(4):398. doi: 10.3390/ph15040398. Pharmaceuticals (Basel). 2022. PMID: 35455395 Free PMC article. Review.
Cited by
-
Case report: Two cases of multiple evanescent white dot syndrome with transient night blindness.Front Ophthalmol (Lausanne). 2025 Feb 17;5:1557294. doi: 10.3389/fopht.2025.1557294. eCollection 2025. Front Ophthalmol (Lausanne). 2025. PMID: 40034237 Free PMC article.
-
Choroidal vascular characteristics in anisometropic amblyopia: a comparative analysis.BMC Ophthalmol. 2025 May 21;25(1):301. doi: 10.1186/s12886-025-04143-3. BMC Ophthalmol. 2025. PMID: 40399854 Free PMC article.
References
-
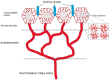
- Fryczkowski AW (1994) Anatomical and functional choroidal lobuli. Int Ophthalmol 18(3):131–41. 10.1007/BF00915961. (PMID: 7852018) - PubMed
-
- Olver JM (1990) Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye (Lond) 4(Pt 2):262–72. 10.1038/eye.1990.38. (PMID: 2379644) - PubMed
-
- Hope-Ross MW (1997) ICG dye: physical and pharmaceutical properties. In: Yannuzzi LA, Flower RW, Slakter JS (eds) Indocyanine Green Angiography. Mosby-Year Book St. Louis, Missouri, pp 46–49
-
- Chang AA, Morse LS, Handa JT, Morales RB, Tucker R, Hjelmeland L, Yannuzzi LA (1998) Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 105(6):1060–8. 10.1016/S0161-6420(98)96008-0. (PMID: 9627657) - PubMed
-
- Herbort CP, Papadia M, Mantovani A (2012) Classification of choroiditis based on inflammatory lesion process rather than fundus appearance: enhanced comprehension through the ICGA concepts of the iceberg and jellyfish effects. Klin Monbl Augenheilkd 229(4):306–13. 10.1055/s-0031-1299394. (Epub 2012 Apr 11 PMID: 22495994) - PubMed
Publication types
LinkOut - more resources
Full Text Sources

