Pelophen B is a non-taxoid binding microtubule-stabilizing agent with promising preclinical anticancer properties
- PMID: 39633082
- PMCID: PMC11618378
- DOI: 10.1038/s41598-024-80672-z
Pelophen B is a non-taxoid binding microtubule-stabilizing agent with promising preclinical anticancer properties
Abstract
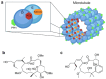
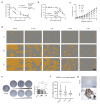
Taxanes, such as paclitaxel (PTX), stabilize microtubules and are used as a first-line therapy in multiple cancer types. Disruption of microtubule equilibrium, which plays an essential role in mitosis and cell homeostasis, ultimately results in cell death. Even though PTX is a very potent chemotherapy, its use is associated with major side effects and therapy resistance. Pelophen B (PPH), a synthetic analog of peloruside A, stabilizes microtubules through interaction with a non-taxoid binding site of β-tubulin. We evaluated the anticancer effect of PPH in a variety of tumor types by using established cell lines, early-passage cultures and ex vivo tumor-derived cultures that preserve the 3D architecture of the tumor microenvironment. PPH significantly blocks colony formation capacity, reduces viability and exerts additivity with PTX. Interestingly, PPH overcomes resistance to PTX. Mechanistically, PPH induces a G2/M cell cycle arrest and increases the presence of tubulin polymerization promoting protein (TPPP), inducing lysine 40 acetylation of α-tubulin. Although, results induced by paclitaxel or PPH are concordant, PPH's unique microtubule binding mechanism enables PTX additivity and ensures overcoming PTX-induced resistance. In conclusion, PPH results in remarkable anti-cancer activity in a range of preclinical models supporting further clinical investigation of PPH as a therapeutic anticancer agent.
Keywords: Breast cancer; Microtubule-stabilizing agent; Ovarian cancer; Paclitaxel; Pelophen B; Sarcoma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Johan Vander Eycken is patentholder of Pelophen B (PCT/EP2014/075903). All other authors declare no competing interests. Ethics approval and consent to participate: Informed consent of patients to use tumor material was obtained after the study protocol was approved by the institutional review board of the Ghent University Hospital (EC 2018/0080 and ONZ-2022–0345, EC 2019/1869 and ONZ-2023-0502). All animal experiments were approved by the local ethics committee (ECD 19-45 and ECD 19-86, Ghent University Hospital). All animal experiments were conducted in accordance with the regulatory guidelines of the Ethics Committee of Ghent University Hospital and the ARRIVE guidelines.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

