Pyroptotic cell corpses are crowned with F-actin-rich filopodia that engage CLEC9A signaling in incoming dendritic cells
- PMID: 39633178
- PMCID: PMC11695261
- DOI: 10.1038/s41590-024-02024-3
Pyroptotic cell corpses are crowned with F-actin-rich filopodia that engage CLEC9A signaling in incoming dendritic cells
Abstract
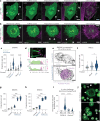
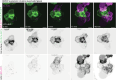
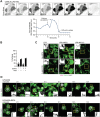
While apoptosis dismantles the cell to enforce immunological silence, pyroptotic cell death provokes inflammation. Little is known of the structural architecture of cells undergoing pyroptosis, and whether pyroptotic corpses are immunogenic. Here we report that inflammasomes trigger the Gasdermin-D- and calcium-dependent eruption of filopodia from the plasma membrane minutes before pyroptotic cell rupture, to crown the resultant corpse with filopodia. As a rich store of F-actin, pyroptotic filopodia are recognized by dendritic cells through the F-actin receptor, CLEC9A (DNGR1). We propose that cells assemble filopodia before cell rupture to serve as a posthumous mark for a cell that has died by gasdermin-induced pyroptosis, or MLKL-induced necroptosis, for recognition by dendritic cells. This study reveals the spectacular morphology of pyroptosis and identifies a mechanism by which inflammasomes induce pyroptotic cells to construct a de novo alarmin that activates dendritic cells via CLEC9A, which coordinates the transition from innate to adaptive immunity1,2.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: K.S., M.H.L. and J.C.K. declare the following competing interests: K.S. is a coinventor on patent applications for NLRP3 inhibitors that have been licensed to Inflazome Ltd. K.S. served on the Scientific Advisory Board of Inflazome (2016–2017) and Quench Bio, USA (2018–2021) and serves on a Scientific Advisory Board for Novartis, Switzerland (since 2020). M.H.L. is an inventor on patents relating to CLEC9A antibodies. J.C.K. consults and holds equity in Corner Therapeutics, Larkspur Biosciences, MindImmune Therapeutics and Neumora Therapeutics; none of these relationships affected this study. The other authors declare no competing interests.
Figures











References
MeSH terms
Substances
Grants and funding
- AI167993/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 2020-225648/Silicon Valley Community Foundation (SVCF)
- FT190100516/Department of Education and Training | Australian Research Council (ARC)
- 2009075/Department of Health | National Health and Medical Research Council (NHMRC)
- DP230100300/Department of Education and Training | Australian Research Council (ARC)
- DK34854/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 DK034854/DK/NIDDK NIH HHS/United States
- LT0006/2022-L/Human Frontier Science Program (HFSP)
- n/a/University of Queensland (UQ)
- GNT113659/Department of Health | National Health and Medical Research Council (NHMRC)
- R01 AI167993/AI/NIAID NIH HHS/United States
- ALTF 491-2022/European Molecular Biology Organization (EMBO)
- DE200101300/Department of Education and Training | Australian Research Council (ARC)
- AI11655/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DE220100823/Department of Education and Training | Australian Research Council (ARC)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

