Muscle tissue transcriptome of F1 Angus-Nellore bulls and steers feedlot finished: impacts on intramuscular fat deposition
- PMID: 39633259
- PMCID: PMC11616301
- DOI: 10.1186/s12864-024-11066-8
Muscle tissue transcriptome of F1 Angus-Nellore bulls and steers feedlot finished: impacts on intramuscular fat deposition
Abstract
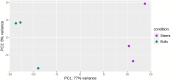
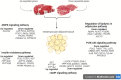
Background: Castration is a common practice in beef cattle production systems to manage breeding and enhance meat quality by promoting intramuscular fat (IMF) deposition, known as marbling. However, the molecular mechanisms that are influenced by castration in beef cattle are poorly understood. The aim of this study was to identify differentially expressed genes (DEGs) and metabolic pathways that regulate IMF deposition in crossbred cattle by RNA sequencing (RNA-Seq) of skeletal muscle tissue. Six hundred and forty F1 Angus-Nellore bulls and steers (n = 320/group) were submitted to feedlot finishing for 180 days. Sixty Longissimus thoracis muscle samples were collected randomly from each group in the hot carcass (at slaughter) and 48 h post-mortem (at deboning), at between 12th and 13th thoracic vertebrae. Three muscle samples of each group were randomly selected for RNA-Seq analysis, while the post-deboning meat samples were submitted to determination of IMF content.
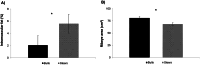
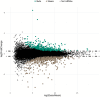
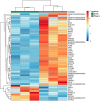
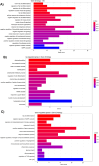
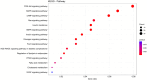
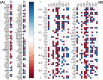
Results: Steers had a 2.7-fold greater IMF content than bulls (5.59 vs. 2.07%; P < 0.01). A total of 921 DEGs (FDR < 0.05) were identified in contrast between Bulls versus Steers; of these, 371 were up-regulated, and 550 were down-regulated. Functional transcriptome enrichment analysis revealed differences in biological processes and metabolic pathways related to adipogenesis and lipogenesis, such as insulin resistance, AMPK, cAMP, regulation of lipolysis in adipocytes, and PI3K-Akt signaling pathways. Candidate genes such as FOXO1, PPARG, PCK2, CALM1, LEP, ADIPOQ, FASN, FABP4, PLIN1, PIK3R3, ROCK2, ADCY5, and ADORA1 were regulated in steers, which explains the expressive difference in IMF content when compared to bulls.
Conclusions: The current findings suggest the importance of these pathways and genes for lipid metabolism in steers with greater IMF. Notably, this study reveals for the first time the involvement of the PI3K-Akt pathway and associated genes in regulating IMF deposition in F1 Angus-Nellore cattle. Castration influenced DEGs linked to energy metabolism and lipid biosynthesis, highlighting key molecular players responsible for IMF accumulation post-castration in beef cattle.
Keywords: Longissimus thoracis; Marbling; RNA sequencing; Sex class.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures followed the ethical guidelines of the Júlio de Mesquita Filho Paulista State University (UNESP), and the protocol was approved by the Animal Use Ethics Committee of the institution (CEUA 07594/2019). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Moreover, informed consent has been obtained from the authority of feedlot facilities of Fazenda Turbilhão, Estrela D’Oeste, São Paulo, Brazil to carry out the study.
Figures
Similar articles
-
Early-life vitamin A supplementation modulates the skeletal muscle transcriptome and intramuscular fat deposition in feedlot-finished beef steers.J Anim Sci. 2025 Jul 31:skaf248. doi: 10.1093/jas/skaf248. Online ahead of print. J Anim Sci. 2025. PMID: 40754836
-
Transcriptome changes associated with fat deposition in the longissimus thoracis of Korean cattle following castration.J Anim Physiol Anim Nutr (Berl). 2020 Nov;104(6):1637-1646. doi: 10.1111/jpn.13393. Epub 2020 Jun 12. J Anim Physiol Anim Nutr (Berl). 2020. PMID: 32533609
-
Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle.PLoS One. 2017 Oct 26;12(10):e0185961. doi: 10.1371/journal.pone.0185961. eCollection 2017. PLoS One. 2017. PMID: 29073274 Free PMC article.
-
TRIENNIAL GROWTH AND DEVELOPMENT SYMPOSIUM: Molecular mechanisms related to bovine intramuscular fat deposition in the longissimus muscle.J Anim Sci. 2017 May;95(5):2284-2303. doi: 10.2527/jas.2016.1160. J Anim Sci. 2017. PMID: 28727015 Review.
-
A review of the role of transcription factors in regulating adipogenesis and lipogenesis in beef cattle.J Anim Breed Genet. 2024 May;141(3):235-256. doi: 10.1111/jbg.12841. Epub 2023 Dec 25. J Anim Breed Genet. 2024. PMID: 38146089 Review.
Cited by
-
Phosphoenolpyruvate carboxykinase 2 (PCK2) attenuates bovine adipocyte lipolysis through PNPLA2 activity repression.Funct Integr Genomics. 2025 Jul 18;25(1):157. doi: 10.1007/s10142-025-01666-2. Funct Integr Genomics. 2025. PMID: 40679709 No abstract available.
-
Current status of transcriptome sequencing technology in ruminants.Front Vet Sci. 2025 Jun 26;12:1558799. doi: 10.3389/fvets.2025.1558799. eCollection 2025. Front Vet Sci. 2025. PMID: 40642274 Free PMC article. Review.
-
Unveiling the Regulatory Mechanism of Tibetan Pigs Adipogenesis Mediated by WNT16: From Differential Phenotypes to the Application of Multi-Omics Approaches.Animals (Basel). 2025 Jun 27;15(13):1904. doi: 10.3390/ani15131904. Animals (Basel). 2025. PMID: 40646803 Free PMC article.
References
-
- Dos Santos MD, Almeida FCR, Silva JM, Costa DS, Souza CN, Santana JL. Rendimento E Acabamento Da carcaça de novilhos inteiros e castrados da raça brangus terminados em confinamento. Revista Brasileira De Higiene E Sanidade Anim. 2014;8(3):62–71.
-
- Santiago BM, Baldassini WA, Chiaratti MR, Pandey AK, Torrecilhas JA, Torres RN, Neto O. R. M. Skeletal muscle gene expression and meat quality of F1 Angus–Nellore young steers and bulls feedlot finished. Livest Sci. 2023a;268:105151.
-
- Moletta JL, Torrecilhas JA, Ornaghi MG, Passetti RAC, Eiras CE, Prado. I.N.d. Feedlot performance of bulls and steers fed on three levels of concentrate in the diets. Acta Scientiarum Anim Sci. 2014;36:323–8.
-
- Seideman SC, Cross HR, Oltjen RR, Schanbacher BD. Utilization of the intact male for red meat production: a review. J Anim Sci. 1982;55:826–40.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

