The impact of a lifestyle promotion program on anthropometric and clinical manifestations in adolescents with polycystic ovarian syndrome: a randomized controlled trial
- PMID: 39633341
- PMCID: PMC11619654
- DOI: 10.1186/s12905-024-03455-8
The impact of a lifestyle promotion program on anthropometric and clinical manifestations in adolescents with polycystic ovarian syndrome: a randomized controlled trial
Abstract
Background: Lifestyle modification can have beneficial effects on improving symptoms of ovary syndrome and anthropometric changes, particularly in obese individuals… However, it is not clear whether these affects in obese adolescents with PCOS are the same as non-PCOS adolescents. We had a study question" Can lifestyle promotion programs, which focus on changing behavioral habits, have an effect on anthropometric parameters and the manifestation of polycystic ovary syndrome (PCOS) in adolescents?"
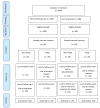
Methods: This was a cluster randomized trial (CRT) that started from January 2021 and follow-up ended in March 2022. 128 participants included adolescent girls (from 14 to 18 years old). The status of PCOS was determined for the participants, following which both the PCOS-afflicted and non-PCOS cohorts were subjected to randomization to either partake in a lifestyle promotion program or to proceed without it. This program included eight sessions that were designed to be implemented for two months. This intervention provides recommendations for a balanced diet and regular exercise, as well as advice on behavior change for adolescents, including those with PCOS, regardless of their weight. All participants were followed up for 12 months and were evaluated at three time points: baseline, 6 and 12 months. Outcomes included changes in the anthropometric indices (weight, hip and waist circumstance), regularity of menstrual cycle, hirsutism score by the modified-Ferriman-Gallwey scale, acne score by the Investigator's Global Assessment Scale, hair loss scores by the Sinclair Graphic Instrument and then physical activity by the Caspian tool and dietary intake status by the Food Frequency Questionnaire. Data was analyzed using the non-parametric Mann-Whitney test for variables with two-time point assessments and generalized estimation equations (GEE) for variables with three time point assessments.
Results: In the study, the intervention group of girls with PCOS exhibited a significant reduction in weight and waist circumference, with an average decrease of 3.14 kg and 4.68 cm, respectively (P < 0.001), compared to the PCOS control group. Similarly, the non-PCOS intervention group showed a decrease in these factors by 2.60 kg and 4.95 cm (P < 0.001) when compared to the non-PCOS control group. After 12 months of intervention, the odds ratio (OR) for menstrual regularity in the PCOS intervention group increased to 3.30 (95% CI: 2.06, 5.25), and the acne score significantly decreased with an OR of 0.46 (95% CI: 0.31, 0.70). In contrast, the non-PCOS intervention group experienced an increase in the OR for menstrual regularity to 2.45 (95% CI: 1.33, 4.25) and an improved in acne score with an OR of 0.44 (95% CI: 0.28, 0.69). No notable differences were observed in the nutritional status among all groups post-intervention. However, a significant increase in physical activity levels, measured in metabolic equivalent minutes per week (met/min/week), was recorded in both intervention groups (p < 0.05).
Conclusion: Manifestations of PCOS in adolescents are improved by a lifestyle promotion program and high schools are considered an appropriate setting to identify those with PCOS and the implementation of a lifestyle modification program. This program was also shown to promote healthy lifestyles for non-PCOS adolescents.
Trial registration: Trial registration number: irct.ir number: IRCT20200114046123N1.
Keywords: Life style; Nutrition assessment; Physical activity; Polycystic ovarian syndrome (PCOS), adolescents, health promotion health evaluation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics and Research Committee of Shahid Beheshti University of Medical Sciences (reference number: IR.SBMU.PHNM.1397.100). We obtained written informed consent from parents of all eligible participants after the study content was clearly explained to the subjects by the trial assistant. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous