The neuropathological basis of elevated serum neurofilament light following experimental concussion
- PMID: 39633506
- PMCID: PMC11619522
- DOI: 10.1186/s40478-024-01883-z
The neuropathological basis of elevated serum neurofilament light following experimental concussion
Abstract
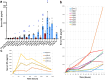
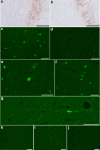
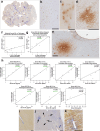
Mild traumatic brain injury (mTBI) or concussion is a substantial health problem globally, with up to 15% of patients experiencing persisting symptoms that can significantly impact quality of life. Currently, the diagnosis of mTBI relies on clinical presentation with ancillary neuroimaging to exclude more severe forms of injury. However, identifying patients at risk for a poor outcome or protracted recovery is challenging, in part due to the lack of early objective tests that reflect the relevant underlying pathology. While the pathophysiology of mTBI is poorly understood, axonal damage caused by rotational forces is now recognized as an important consequence of injury. Moreover, serum measurement of the neurofilament light (NfL) protein has emerged as a potentially promising biomarker of injury. Understanding the pathological processes that determine serum NfL dynamics over time, and the ability of NfL to reflect underlying pathology will be critical for future clinical research aimed at reducing the burden of disability after mild TBI. Using a gyrencephalic model of head rotational acceleration scaled to human concussion, we demonstrate significant elevations in serum NfL, with a peak at 3 days post-injury. Moreover, increased serum NfL was detectable out to 2 weeks post-injury, with some evidence it follows a biphasic course. Subsequent quantitative histological examinations demonstrate that axonal pathology, including in the absence of neuronal somatic degeneration, was the likely source of elevated serum NfL. However, the extent of axonal pathology quantified via multiple markers did not correlate strongly with the extent of serum NfL. Interestingly, the extent of blood-brain barrier (BBB) permeability offered more robust correlations with serum NfL measured at multiple time points, suggesting BBB disruption is an important determinant of serum biomarker dynamics after mTBI. These data provide novel insights to the temporal course and pathological basis of serum NfL measurements that inform its utility as a biomarker in mTBI.
Keywords: Blood brain barrier; Concussion; Diffuse axonal injury; Mild traumatic brain injury; Neurofilament light; Serum biomarkers.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All in vivo experiments were conducted in accordance with protocols approved by The University of Pennsylvania Institutional Animal Care and Use Committee. Consent for publication: Not applicable. Competing interests: Dr. Ramon Diaz-Arrastia has consulted with MesoScale Discoveries and BrainBox Solutions, Inc. Dr. Douglas Smith has consulted with Abbott Laboratories.
Figures


References
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR (1989) Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 15:49–59 - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12:557–563 - PubMed
-
- Al Nimer F, Thelin E, Nystrom H, Dring AM, Svenningsson A, Piehl F, Nelson DW, Bellander BM (2015) comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS ONE 10:e0132177. 10.1371/journal.pone.0132177 - PMC - PubMed
-
- Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonniere L, Larochelle C, Prat A (2015) Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis 74:14–24. 10.1016/j.nbd.2014.09.016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

