Reduced GATA1 levels are associated with ineffective erythropoiesis in sickle cell anemia
- PMID: 39633531
- PMCID: PMC12050926
- DOI: 10.3324/haematol.2024.286010
Reduced GATA1 levels are associated with ineffective erythropoiesis in sickle cell anemia
Abstract
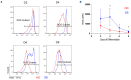
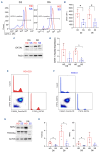
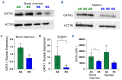
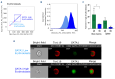
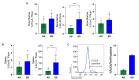
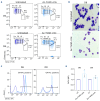
Ineffective erythropoiesis (IE) is defined as the abnormal differentiation and excessive destruction of erythroblasts i n the bone marrow, accompanied by an expanded progenitor compartment and relative reduction in the production of reticulocytes. It is a defining feature of many types of anemia, including β-thalassemia. GATA1 is an essential transcription factor for erythroid differentiation, known to be implicated in hematological conditions presenting with IE, including β-thalassemia and congenital dyserythropoietic anemia. However, little is known about the role of GATA1 in the erythropoietic defects recently described in sickle cell anemia (SCA). In the present study, we performed a detailed characterization of the role of GATA1 and ineffective erythropoiesis in SCA using both in vitro and in vivo assay systems. We demonstrate a significant decrease in GATA1 protein levels during SCA erythropoiesis and a concomitant increase in oxidative stress. Furthermore, we found that an increase in the activity of the inflammatory caspase, caspase 1, was driving the decrease in GATA1 levels during SCA erythropoiesis and that, upon inhibition of caspase 1 activity, SCA erythropoiesis was rescued and GATA1 levels partially restored. Our study further elucidates the defect in erythropoiesis in SCA, and may therefore help in the development of novel approaches to normalize the bone marrow niche prior to stem cell transplantation, or facilitate the production of healthy stem cells for gene therapy.
Figures


References
-
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood. 2005;105(3):921-923. - PubMed
-
- Arlet JB, Ribeil JA, Guillem F, et al. . HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in betathalassaemia. Nature. 2014;514(7521):242-246. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

