SGK1-Mediated Vascular Smooth Muscle Cell Phenotypic Transformation Promotes Thoracic Aortic Dissection Progression
- PMID: 39633576
- PMCID: PMC11748913
- DOI: 10.1161/ATVBAHA.124.321421
SGK1-Mediated Vascular Smooth Muscle Cell Phenotypic Transformation Promotes Thoracic Aortic Dissection Progression
Abstract
Background: The occurrence of thoracic aortic dissection (TAD) is closely related to the transformation of vascular smooth muscle cells (VSMCs) from a contractile to a synthetic phenotype. The role of SGK1 (serum- and glucocorticoid-regulated kinase 1) in VSMC phenotypic transformation and TAD occurrence is unclear.
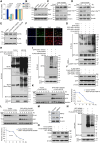
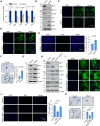
Methods: Four-week-old male Sgk1F/F (Sgk1 floxed) and Sgk1F/F;TaglnCre (smooth muscle cell-specific Sgk1 knockout) mice were administered β-aminopropionitrile monofumarate for 4 weeks to model TAD. The SGK1 inhibitor GSK650394 was administered daily via intraperitoneal injection to treat the mouse model of TAD. Immunopurification and mass spectrometry were used to identify proteins that interact with SGK1. Immunoprecipitation, immunofluorescence colocalization, and GST (glutathione S-transferase) pull-down were used to detect molecular interactions between SGK1 and SIRT6 (sirtuin 6). RNA-sequencing analysis was performed to evaluate changes in the SIRT6 transcriptome. Quantitative chromatin immunoprecipitation was used to determine the target genes regulated by SIRT6. Functional experiments were also conducted to investigate the role of SGK1-SIRT6-MMP9 (matrix metalloproteinase 9) in VSMC phenotypic transformation. The effect of SGK1 regulation on target genes was evaluated in human and mouse TAD samples.
Results: Sgk1F/F;TaglnCre or pharmacological blockade of Sgk1 inhibited the formation and rupture of β-aminopropionitrile monofumarate-induced TADs in mice and reduced the degradation of the ECM (extracellular matrix) in vessels. Mechanistically, SGK1 promoted the ubiquitination and degradation of SIRT6 by phosphorylating SIRT6 at Ser338, thereby reducing the expression of the SIRT6 protein. Furthermore, SIRT6 transcriptionally inhibits the expression of MMP9 through epigenetic modification, forming the SGK1-SIRT6-MMP9 regulatory axis, which participates in the ECM signaling pathway. Additionally, our data showed that the lack of SGK1-mediated inhibition of ECM degradation and VSMC phenotypic transformation is partially dependent on the regulatory effect of SIRT6-MMP9.
Conclusions: These findings highlight the key role of SGK1 in the pathogenesis of TAD. A lack of SGK1 inhibits VSMC phenotypic transformation by regulating the SIRT6-MMP9 axis, providing insights into potential epigenetic strategies for TAD treatment.
Keywords: dissection, thoracic aorta; mass spectrometry; sequence analysis, RNA; sirtuins; ubiquitination.
Conflict of interest statement
None.
Figures






References
-
- Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–348. doi: 10.1038/s41569-020-00472-6 - PubMed
-
- Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med. 2002;346:1484–1486. doi: 10.1056/NEJM200205093461910 - PubMed
-
- El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–786. doi: 10.1038/nrcardio.2009.191 - PubMed
-
- Jauhiainen S, Kiema M, Hedman M, Laakkonen JP. Large vessel cell heterogeneity and plasticity: focus in aortic aneurysms. Arterioscler Thromb Vasc Biol. 2022;42:811–818. doi: 10.1161/ATVBAHA.121.316237 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

