Research progress on FSH-FSHR signaling in the pathogenesis of non-reproductive diseases
- PMID: 39633710
- PMCID: PMC11615068
- DOI: 10.3389/fcell.2024.1506450
Research progress on FSH-FSHR signaling in the pathogenesis of non-reproductive diseases
Abstract
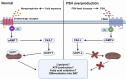
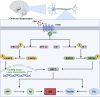
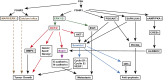
Follicle-stimulating hormone (FSH), a glycoprotein hormone synthesized and secreted by the anterior pituitary gland, plays a critical role in reproductive development and regulation by binding to FSH receptor (FSHR). Beyond reproductive tissue, FSHRs have been identified in various non-reproductive tissues, indicating broader functions. FSH levels chronically rise during menopause and remain elevated in postmenopausal life. This increase in FSH level has been indicated to be associated with heightened risk of several non-reproductive diseases, including osteoporosis, hypercholesterolemia, type 2 diabetes mellitus, obesity, cardiovascular disease, Alzheimer's disease, and certain cancers. In this review, we will examine the role of FSH-FSHR signaling in the pathogenesis of these non-reproductive diseases and explore therapeutic strategies targeting FSH-FSHR signaling pathways.
Keywords: FSH; FSHR; menopause; non-reproductive diseases; reproduction.
Copyright © 2024 Li, Ling and Kuang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

