The perspectives of broadband metasurfaces and photo-electric tweezer applications
- PMID: 39633930
- PMCID: PMC11501245
- DOI: 10.1515/nanoph-2021-0711
The perspectives of broadband metasurfaces and photo-electric tweezer applications
Abstract
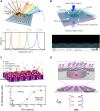
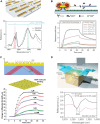
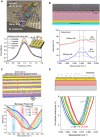
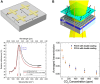
With strong demands of real-time monitoring of biomolecules or environmental pollutants, overcoming technical hurdles on control and detection of freely diffusive nanoscale objects become a question of issue to solve in a variety of research fields. Most existing optical techniques inevitably require labeling to the target material, which sometimes denature the measuring biomaterials. For highly efficient real-time monitoring without complicated pretreatment or labeling, many successes in development of label-free or non-destructive detection techniques via increased sensitivity were accomplished by the additional structures. Metasurface-based two-dimensional photonic/electric devices have recently represented extraordinary performances in both manipulation and sensing for various small particles and biochemical species, repeatedly overcoming the limit of detection achieved right before. In parallel, various metasurface-based devices were also introduced promoting transportation of targets into optical hotspot sites, overcoming diffusion limits. We noted this point, therefore, reviewed two major research fields such as metasurface-assisted material sensing and transportation technologies that have contributed to present prospective sensing technologies, then showed perspective views on how great synergy can be created when two technologies are cleverly integrated. Recently, a trend of conceptual merging of optical detection and transporting schemes beyond both diffraction limit and diffusion limit leads to a creation of exceptional performance in molecular detections. In this review, the trends of the latest technologies accomplishing this purpose by hybridization of various composite materials and functional metasurfaces will be introduced.
Keywords: biosensor; dielectrophoresis; metasurface; optical sensing; plasmonic trapping; tweezer.
© 2022 Geon Lee et al., published by De Gruyter, Berlin/Boston.
Figures







References
-
- Romano S., Zito G., Torino S., et al. Label-free sensing of ultralow-weight molecules with all-dielectric metasurfaces supporting bound states in the continuum. Photon. Res. . 2018;6:726. doi: 10.1364/prj.6.000726. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
