Label-Free Anti-Brownian Trapping of Single Nanoparticles in Solution
- PMID: 39634022
- PMCID: PMC11613540
- DOI: 10.1021/acs.jpcc.4c05878
Label-Free Anti-Brownian Trapping of Single Nanoparticles in Solution
Abstract
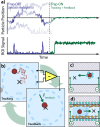
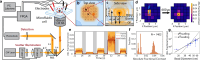
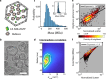
Today, biomolecular nanoparticles are prevalent as diagnostic tools and molecular delivery carriers, and it is particularly useful to examine individuals within a sample population to quantify the variations between objects and directly observe the molecular dynamics involving these objects. Using interferometric scattering as a highly sensitive label-free detection scheme, we recently developed the interferometric scattering anti-Brownian electrokinetic (ISABEL) trap to hold a single nanoparticle in solution for extended optical observation. In this perspective, we describe how we implemented this trap, how it extends the capabilities of previous ABEL traps, and how we have begun to study individual carboxysomes, a fascinating biological carbon fixation nanocompartment. By monitoring single nanocompartments for seconds to minutes in the ISABEL trap using simultaneous interferometric scattering and fluorescence spectroscopy, we have demonstrated single-compartment mass measurements, cargo-loading trends, and redox sensing inside individual particles. These experiments benefit from rich multiplexed correlative measurements utilizing both scattering and fluorescence with many exciting future capabilities within reach.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Kirst H.; Ferlez B. H.; Lindner S. N.; Cotton C. A. R.; Bar-Even A.; Kerfeld C. A. Toward a glycyl radical enzyme containing synthetic bacterial microcompartment to produce pyruvate from formate and acetate. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2116871119 10.1073/pnas.2116871119. - DOI - PMC - PubMed
-
- Aharonovich I.; Castelletto S.; Simpson D. A.; Su C. H.; Greentree A. D.; Prawer S. Diamond-based single-photon emitters. Rep. Prog. Phys. 2011, 74, 076501 10.1088/0034-4885/74/7/076501. - DOI
LinkOut - more resources
Full Text Sources
