Two-dimensional metal halide perovskites and their heterostructures: from synthesis to applications
- PMID: 39634119
- PMCID: PMC11501535
- DOI: 10.1515/nanoph-2022-0797
Two-dimensional metal halide perovskites and their heterostructures: from synthesis to applications
Abstract
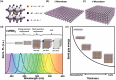
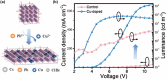
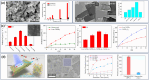
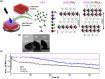
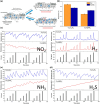
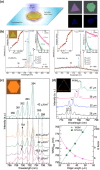
Size- and shape-dependent unique properties of the metal halide perovskite nanocrystals make them promising building blocks for constructing various electronic and optoelectronic devices. These unique properties together with their easy colloidal synthesis render them efficient nanoscale functional components for multiple applications ranging from light emission devices to energy conversion and storage devices. Recently, two-dimensional (2D) metal halide perovskites in the form of nanosheets (NSs) or nanoplatelets (NPls) are being intensively studied due to their promising 2D geometry which is more compatible with the conventional electronic and optoelectronic device structures where film-like components are usually employed. In particular, 2D perovskites exhibit unique thickness-dependent properties due to the strong quantum confinement effect, while enabling the bandgap tuning in a wide spectral range. In this review the synthesis procedures of 2D perovskite nanostructures will be summarized, while the application-related properties together with the corresponding applications will be extensively discussed. In addition, perovskite nanocrystals/2D material heterostructures will be reviewed in detail. Finally, the wide application range of the 2D perovskite-based structures developed to date, including pure perovskites and their heterostructures, will be presented while the improved synergetic properties of the multifunctional materials will be discussed in a comprehensive way.
Keywords: 2D materials; energy conversion; energy storage; perovskite nanocrystals; photodetectors; sensing; synthesis routes.
© 2023 the author(s), published by De Gruyter, Berlin/Boston.
Figures
































References
-
- Kostopoulou A., Brintakis K., Nasikas N. K., Stratakis E. Perovskite nanocrystals for energy conversion and storage. Nanophotonics . 2019;8(10):1607–1640. doi: 10.1515/nanoph-2019-0119. - DOI
-
- Kostopoulou A., Kymakis E., Stratakis E. Perovskite nanostructures for photovoltaic and energy storage devices. J. Mater. Chem. A . 2018;6(21):9765–9798. doi: 10.1039/C8TA01964A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
