A Glucose-Responsive Glucagon-Micelle for the Prevention of Hypoglycemia
- PMID: 39634211
- PMCID: PMC11613347
- DOI: 10.1021/acscentsci.4c00937
A Glucose-Responsive Glucagon-Micelle for the Prevention of Hypoglycemia
Abstract
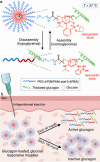
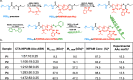
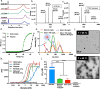
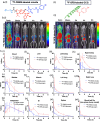
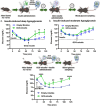
While glucose-responsive insulin delivery systems are in widespread clinical use to treat insulin insufficiency, the on-demand supplementation of glucagon for acute hypoglycemia treatment remains understudied. A self-regulated glucagon release material is highly desired to mitigate the potential risks of severe insulin-induced hypoglycemia. Here, we describe a glucose-responsive polymeric nanosystem with glucagon covalently grafted to the end-group. Under normoglycemic conditions, phenylboronic acid units in the polymer chain reversibly bind glucose, triggering self-assembly of the conjugate into micelles. During hypoglycemia, however, the micelle disassembles into its original, unimeric state, revealing the active glucagon conjugate. The formulation showed a 5-fold increase in activity compared to native glucagon when tested in vitro. Glucagon-loaded micelles injected into mice prevented or reversed deep hypoglycemia when administered prior to or during an insulin challenge. Glucagon release was only observed at or below the counterregulatory threshold and not during normoglycemia or moderate hypoglycemia. The in vivo acute and chronic toxicity analysis, along with μPET/μCT imaging, established the biosafety profile of this formulation and demonstrated no organ accumulation. This proof-of-concept work is the first step toward development of a translational, stimuli-responsive glucagon delivery platform to control glycemia.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Ong K. L.; Stafford L. K.; Vos T.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402 (10397), 203–234. 10.1016/S0140-6736(23)01301-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
