A clinical-radiomics nomogram based on multisequence MRI for predicting the outcome of patients with advanced nasopharyngeal carcinoma receiving chemoradiotherapy
- PMID: 39634263
- PMCID: PMC11615067
- DOI: 10.3389/fonc.2024.1460426
A clinical-radiomics nomogram based on multisequence MRI for predicting the outcome of patients with advanced nasopharyngeal carcinoma receiving chemoradiotherapy
Abstract
Problem: Nasopharyngeal carcinoma (NPC) is a common malignant tumor with high heterogeneity and is mainly treated with chemoradiotherapy. It is important to predict the outcome of patients with advanced NPC after chemoradiotherapy to devise customized treatment strategies. Traditional MRI methods have limited predictive power, and better predictive models are needed.
Aim: To evaluate the predictive value of a clinical-radiomics nomogram based on multisequence MRI in predicting the outcome of advanced NPC patients receiving chemoradiotherapy.
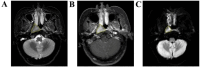
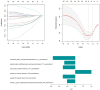
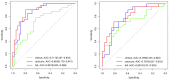
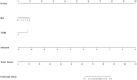
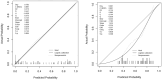
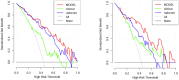
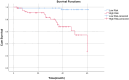
Methods: This prospective study included a retrospective analysis of 118 patients with advanced NPC who underwent MRI prior to chemoradiotherapy. The primary endpoint was progression-free survival (PFS). The maximum ROIs of lesions at the same level were determined via axial T2-weighted imaging short-time inversion recovery (T2WI-STIR), contrast-enhanced T1-weighted imaging (CE-T1WI), and diffusion-weighted imaging (DWI) with solid tumor components, and the radiomic features were extracted. After feature selection, the radiomics score was calculated, and a nomogram was constructed combining the radiomics score with the clinical features. The diagnostic efficacy of the model was evaluated by the area under the receiver operating characteristic curve (AUC), and the clinical application value of the nomogram was evaluated by decision curve analysis (DCA) and a correction curve. Patients were divided into a high-risk group and a low-risk group, and the median risk score calculated by the joint prediction model was used as the cutoff value. Kaplan-Meier analysis and the log-rank test were used to compare the differences in survival curves between the two groups.
Results: The AUCs of the nomogram model constructed by the combination of the radiomics score and neutrophil-to-lymphocyte ratio (NLR) and T stage in the training group and validation group were 0.897 (95% CI: 0.825-0.968) and 0.801 (95% CI: 0.673-0.929), respectively. Kaplan-Meier survival analysis demonstrated that the model effectively stratified patients into high- and low-risk groups, with significant differences in prognosis.
Conclusion: This clinical-radiomics nomogram based on multisequence MRI offers a noninvasive, effective tool for predicting the outcome of advanced NPC patients receiving chemoradiotherapy, promoting individualized treatment approaches.
Keywords: magnetic resonance imaging; nasopharyngeal carcinoma; nomogram; radiomics; survival models.
Copyright © 2024 Chen, Wang, Meng, Zhao, Wang, Zhang and Zhou.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Mohammed MA, Ghani MKA, Hamed RI, Ibrahim DA. Analysis of an electronic methods for nasopharyngeal carcinoma: Prevalence, diagnosis, challenges and technologies. J Comput Sci. (2017) 21:241–54. doi: 10.1016/j.jocs.2017.04.006 - DOI
-
- Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources

