Mesenchymal GDNF promotes intestinal enterochromaffin cell differentiation
- PMID: 39634560
- PMCID: PMC11616604
- DOI: 10.1016/j.isci.2024.111246
Mesenchymal GDNF promotes intestinal enterochromaffin cell differentiation
Abstract
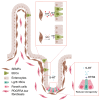
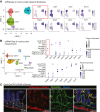
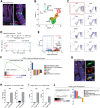
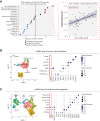
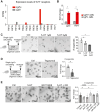
Enteroendocrine cells (EECs) differentiate and mature to form functionally distinct populations upon migration along the intestinal crypt-villus axis, but how niche signals affect this process is poorly understood. Here, we identify expression of Glial cell line-derived neurotrophic factor (GDNF) in the intestinal subepithelial myofibroblasts (SEMFs), while the GDNF receptor RET was expressed in a subset of EECs, suggesting GDNF-mediated regulation. Indeed, GDNF-RET signaling induced increased expression of EEC genes including Tph1, encoding for the rate-limiting enzyme for 5-hydroxytryptamine (5-HT, serotonin) biosynthesis, and increased the frequency of 5-HT+ enterochromaffin cells (ECs) in mouse organoid culture experiments and in vivo. Moreover, expression of the 5-HT receptor Htr4 was enriched in Lgr5+ intestinal stem cells (ISCs) and 5-HT reduced the ISC clonogenicity. In summary, our results show that GDNF-RET signaling regulate EEC differentiation, and suggest 5-HT as a potential niche factor regulating Lgr5+ ISC activity, with potential implications in intestinal regeneration.
Keywords: Biological sciences; Molecular network; Molecular physiology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

