Atomistic Multiscale Modeling of Colloidal Plasmonic Nanoparticles
- PMID: 39634643
- PMCID: PMC11613212
- DOI: 10.1021/acsphyschemau.4c00052
Atomistic Multiscale Modeling of Colloidal Plasmonic Nanoparticles
Abstract
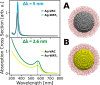
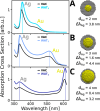
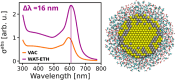
A novel fully atomistic multiscale classical approach to model the optical response of solvated real-size plasmonic nanoparticles (NPs) is presented. The model is based on the coupling of the Frequency Dependent Fluctuating Charges and Fluctuating Dipoles (ωFQFμ), specifically designed to describe plasmonic substrates, and the polarizable Fluctuating Charges (FQ) classical force field to model the solvating environment. The resulting ωFQFμ/FQ approach accounts for the interactions between the radiation and the NP, as well as with the surrounding solvent molecules, by incorporating mutual interactions between the plasmonic substrate and solvent. ωFQFμ/FQ is validated against reference TD-DFTB/FQ calculations, demonstrating remarkable accuracy, particularly in reproducing plasmon resonance frequency shifts for structures below the quantum-size limit. The flexibility and reliability of the approach are also demonstrated by simulating the optical response of homogeneous and bimetallic NPs dissolved in pure solvents and solvent mixtures.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Dutta A.; Medda A.; Patra A. Recent advances and perspectives on colloidal semiconductor nanoplatelets for optoelectronic applications. J. Phys. Chem. C 2021, 125, 20–30. 10.1021/acs.jpcc.0c09416. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
