SOPRANO: Macitentan in patients with pulmonary hypertension following left ventricular assist device implantation
- PMID: 39635465
- PMCID: PMC11615754
- DOI: 10.1002/pul2.12446
SOPRANO: Macitentan in patients with pulmonary hypertension following left ventricular assist device implantation
Abstract
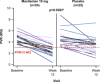
Macitentan is a dual endothelin receptor antagonist (ERA) approved for treating pulmonary arterial hypertension (PAH). SOPRANO evaluated the efficacy and safety of macitentan versus placebo in pulmonary hypertension (PH) patients after left ventricular assist device (LVAD) implantation. SOPRANO was a phase 2, multicenter, double-blind, randomized, placebo-controlled, parallel-group study. Patients with an LVAD implanted within the prior 90 days who had persistent PH (i.e., mean pulmonary arterial pressure ≥25 mmHg, pulmonary artery wedge pressure [PAWP] ≤18 mmHg, and pulmonary vascular resistance [PVR] >3 Wood units [WU]) were randomized (1:1) to macitentan 10 mg or placebo once daily for 12 weeks. The primary endpoint was change in PVR. Secondary endpoints included change in right-heart catheterization hemodynamic variables, N-terminal prohormone of brain natriuretic peptide levels, World Health Organization functional class, and safety/tolerability. Fifty-seven patients were randomized to macitentan (n = 28) or placebo (n = 29). A statistically significant reduction in PVR from baseline to Week 12 was observed with macitentan versus placebo (placebo-corrected geometric mean ratio, 0.74; 95% confidence interval, 0.58-0.94; p = .0158). No statistically significant differences were observed in secondary endpoints. In a post-hoc analysis, 66.7% of patients receiving macitentan achieved PVR <3 WU versus 40.0% receiving placebo (p = .0383). Macitentan was generally well tolerated; adverse events were consistent with those in previous PAH studies with macitentan. In conclusion, macitentan showed promising tolerability and significantly reduced PVR in PH patients with persistently elevated PVR after LVAD implantation. ClinicalTrials. gov identifier: NCT02554903.
Keywords: SOPRANO; left ventricular assist device; macitentan; pulmonary hypertension; pulmonary vascular resistance.
© 2024 Actelion Pharmaceuticals US, Inc. Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Robert P. Frantz is receiving grants and research support from United Therapeutics, Medtronic, and Gossamer Bio, and is scientific medical advisor to Altavant, ShouTi, Liquidia Corporation, Merck, Tenax Therapeutics, and Janssen Pharmaceutical Companies of Johnson & Johnson. His institution has received funding from Bayer and Gossamer Bio. Shashank S. Desai has served on the speakers’ bureau for Abbott. Gregory Ewald is on the speakers’ bureau and is a consultant with Abbott. Veronica Franco's institution receives research support from Acceleron/Merck, Gossamer, Janssen, United Therapeutics, Aerovate Therapeutics, Respira, and Cereno Scientific. Antoine Hage is receiving grants and research support from United Therapeutics, Lung LLC, Arena, Reata, and Bayer, and is a stockholder in Johnson & Johnson and Pfizer. Evelyn M. Horn's institution has received funding from Gossamer, Acceleron/Merck, Abbott, and Cereno Scientific. Stacy Mandras is a speaker and consultant for United Therapeutics and Bayer Pharmaceuticals. Myung H. Park is a scientific medical advisor with AstraZeneca. Ashwin K. Ravichandran is consulting/speaking for Abbott, Medtronic and speaking for United Therapeutics, Janssen, and Bayer; he personally receives no grants from these companies, but his institution does. Ronald Zolty is a consultant for Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, United Therapeutics, and Alnylam. Mark A. Rocco, Mona Selej, and Carol Zhao were employees of Actelion Pharmaceuticals US, Inc., South San Francisco, CA (at the time of manuscript development) and are current stockholders of Johnson & Johnson. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind M, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson U, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics S. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):139. - PubMed
-
- Mancini D, Colombo PC. Left ventricular assist devices: a rapidly evolving alternative to transplant. J Am Coll Cardiol. 2015;65(23):2542–2555. - PubMed
-
- King PM, Raymer DS, Shuster J, Crain M, Bhatia A, Hartupee J, Schilling JD. Right heart failure while on left ventricular assist device support is associated with primary graft dysfunction. ASAIO J. 2020;66(10):1137–1141. - PubMed
-
- Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren E, Zuckermann A, International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation C . The 2016 international society for heart lung transplantation listing criteria for heart transplantation: A 10‐year update. J Heart and Lung Transpl. 2016;35(1):1–23. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

